Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Parvaneh Shafieyoona, Maryam Salahvarzia and Ebrahim Mehdipoura*
Received: November 30, 2022; Published: December 08, 2022
*Corresponding author: Ebrahim Mehdipoura, Department of Chemistry, Faculty of Science, Lorestan University, Khorramabad, Iran.
DOI: 10.26717/BJSTR.2022.47.007517
An experimental and theoretical new investigation has been reported on glyoxal bis (N-phenyl) osazone (GPO) as a ligand and [glyoxal bis (N-phenyl) osazone] palladium (GPOP) as a complex. GPOP was prepared from the reaction of palladium (II) chloride and glyoxal bis (N-phenyl) osazone as a free ligand (GPO) in two steps. Complex (GPOP) synthesis for the first time was confirmed using IR and 1H-NMR spectra. The catalytic activity of this complex exhibited as a novel catalyst in Heck reactions in good yield. The products were characterized using FT- IR and 1H-NMR spectra. The optimized geometry, HOMO-LUMO analysis, and Molecular Electrostatic Potential studies of the GPO have been investigated using the DFT method. Molecular Docking studies of GPOP have also been reported to present the biological properties of the palladium complex.
Keywords: [glyoxal bis (N-phenyl) osazone] palladium; Heck reaction; glyoxal bis (N-phenyl) osazone. MEP; HOMO-LUMO; Molecular docking
Abbreviations: GPO: Glyoxal Bis (N-Phenyl) Osazone; GPOP: Glyoxal Bis (N-Phenyl) Osazone Palladium; HOMO: Highest Occupied Molecular Orbital; LUMO: Lowest Unoccupied Molecular Orbital; MEP: Molecular Electrostatic Potential; DFT: Density Functional Theory; PASS: Prediction of Activity Spectra; LGA: Lamarckian Genetic Algorithm
The Mizoroki-Heck as a cross-coupling method is one of the important reactions for Carbon-Carbon bond formation. This reaction permits the substitution reactions on the planar sp2-hybridized carbon atom. The Heck reaction was described in the presence of an organopalladium catalyst. The unsaturated halide and the large variety of olefin derivatives can be used that contain at least one hydrogen, in the presence of a base, are applied for the Mizoroki-Heck coupling reaction [1-4]. These reactions are performed by coordination and migratory insertion of the olefin derivatives to the palla dium metal. Then, the bond rotates and places the two groups trans to each other to regard the steric strain and subsequent β-hydride elimination results in a final product. Based on the reductive elimination regenerates the palladium (0) catalyst. Heck’s methodology is excellent because of its high chemoselectivity and mild condition. It is known that Palladium complexes are versatile and nontoxic. On the other hand, applying potent and efficient catalysts plays a significant role in synthetic chemistry. In this line, some of the ligands contain nitrogen atoms as an excellent class of interesting compounds such as bipyridine [5], sulfonamide [6,7], and thiourea [8] which have been employed. Palladium–catalyzed coupling reactions are also applied in natural products and drugs [9-12]. It has been accepted that changing the structural parts of this class of ligand contains nitrogen atoms that are diimine (LH2) derivatives.
Due to the lone pairs of the nitrogen atoms and the π-electron of the C=N bond, these ligands act as efficient electron donors. Osazone compounds as an efficient ligand were formed from α-dicarbonyl compounds with an excess of the hydrazine derivatives. Osazone derivatives are a group of organic compounds that have diimines fragments [13-18] Glyoxal bishydrazones and pyridyl-hydrazone were prepared and examined as a ligand for the Mizoroki- Heck cross-coupling reaction of aryl halides, and olefin was reported [19,20]. These results prompted us to synthesize a novel nontoxic osazone derivative for the Mizoroki-Heck coupling reaction. Many critical efforts have been made to develop versatile ligands and palladium catalysts for these types of reactions. In our previous report, related to the palladium complex, its crystal structure and mixed ligand were induced [21-24]. In this research, in addition to the catalytic property of Pd complex (GPOP), its biological properties have been studied using computational methods. In this study, the electronic properties and HOMO (highest occupied molecular orbital)-LUMO (lowest unoccupied molecular orbital), and molecular electrostatic potential (MEP) are performed [25]. With attention to the above-mentioned points, studying the interaction of GPOP and HSA, DNA and 2JVU are very attractive for researchers.
In this work, we investigated the interaction of GPOP with HSA and DNA as anticancer and 2JVU as antibacterial agents using molecular docking methods to evaluate the medicinal properties of GPOP. In continuation, molecular docking is applied for the biological activity of the palladium complex.
Physical Measurements and Materials
Phenyl hydrazine, glyoxal, palladium chloride, dichloromethane, and methanol were purchased from Merck and Sigma–Aldrich companies and were used without further purification. Fourier transform infrared (FT-IR) spectra of prepared compounds were recorded at 400–4000 cm−1 region using KBr pellets on Shimadzu FT-IR 8400 spectrometer. 1H-NMR spectrum was recorded on a Brucker Ultrashield 400 MHz spectrometer using DMSO-d6 as solvent and tetramethylsilane as internal standard.
Synthesis of Compounds
The free ligand (GPO with chemical formula C14H14N4) and its complex (GPOP) with chemical formula C14H14N4PdCl2) were syn thesized according to the reported procedure in the next section. IR and 1H-NMR spectroscopy confirmed the structure and purity of the compounds.
Preparation of Glyoxal Bis (N-Phenyl) Osazone (GPO): The synthetic method for preparation of this ligand (GPO) is as follows: 2mL (20mmol) phenyl hydrazine, 0.446mL (10 mmol) 40% aqueous solution of glyoxal, and 30mL methanol used as solvent were placed in a 100mL round-bottomed flask, equipped with a magnetic stirrer. The reaction mixture was stirred for 15 min at room temperature and several drops of acetic acid glacial were added. The reaction mixture was stirred for 4 h at room temperature. After the reaction time, the yellow precipitate appeared. The product was then filtered, washed with methanol (3×10mL), dried in the air, and used without further purification. The obtained product was analyzed without further purification. (Yield 2.42 g, 90.0%, m.p. 169.5°C). IR (KBr, Cm-1): 3304(m), 3032(m), 1597(s), 1564(s),1504(s), 1456(s), 1438(m), 1251(s),1118(s), 1066(m). 1H-NMR (DMSO-d6, ppm): 10.37 (S, 2H, N-H), 7.62 (s, 2H, N=CH), 7.20 (t, 4H, Ph), 6.95 (d, 4H, Ph), and 6.73 (t, 2H, Ph).
Preparation Of [Glyoxal Bis (N-Phenyl) Osazone] Palladium Complex: The synthetic method for preparation of palladium compound is as follows: 0.09 g PdCl2 (0.5mmol) and CH3CN (35mL) were placed in a 100mL round-bottomed flask equipped with a reflux condenser and a magnetic stirrer. The mixture was stirred and warmed to 70 °C to give a light orange solution. Then, 0.119 g ligand (0.5mmol) in 20 mL CH2Cl2 slowly was added. The reaction mixture was stirred under an atmosphere of argon, for 5 h. After the reaction time, the red solution appeared. The solvent was evaporated, and the solid product was obtained and washed with CH- 2Cl2 (3×10 mL). (Yield 0.19 g, 93.0%, m.p. 192°C). IR (KBr, Cm-1): 3275(s), 3053(m), 1593(m), 1564(m), 1496(s), 1218(m), 1251(m), 1116(m), 1068(m). 1H-NMR (DMSO-d6, ppm): 10.38 (S, 2H, N-H), 7.68 (s, 2H, N=CH), 7.27 (t, 4H, Ph), 7.01 (d, 4H, Ph), and 6.78 (t, 2H, Ph).
General Procedure for Heck Reaction: A 100 mL round-bottomed flask was charged with iodobenzene 1mL (10mmol), methyl methacrylate 3mL (30mmol), and using DMF as a solvent (100mL), Then Na2CO3 as base 1.5 gr (15mmol) and catalyst (0.5mol %) were added. The stirring solution was refluxed at 80 °C for 24 h (Scheme 1). After the required time, the reaction mixture was cooled to room temperature and the palladium catalyst was separated from the mixture by filtration. The solution was diluted with water (30 mL) and the product was extracted with ethyl acetate (3×10 mL). Then, it was concentrated under reduced pressure. The organic layer is recrystallized with ethanol and water (1:1).
Computational Details
Density functional theory (DFT) studies on the glyoxal bis (N-phenyl) osazone (GPO) to provide information with higher accuracy, have not yet been reported. Calculations of the GPO were performed using Gaussian 09 software [26]. These calculations include geometry optimization, MEP, and HOMO–LUMO analysis were performed using density functional theory (DFT) [27,28]. PASS (Prediction of Activity Spectra) [29] was used to predict the activity of the ligand. Molecular docking simulation has recently been used as a significant tool to get insight into ligand-receptor interaction and screen molecules for the binding affinities against a special receptor. Molecular docking calculations were performed on Auto Dock software [30]. The output of geometry optimization (using B3LYP/6-311G (p,d) level) for GPOP was used as the input of ligand for docking processes. Lamarckian Genetic Algorithm (LGA) accessible in Auto dock was applied for docking, as the most popular algorithm [31,32]. The 3D crystal structure of employed DNA, Escherichia coli, and HSA as receptors were obtained from Protein Data Bank (PDB ID: 423D, 2JVU, 1AO6).
Synthesis and Characterization
The synthetic route of GPO as free ligand and GPOP as complex, which was previously reported in experimental are shown in (Schemes 2-4).
Molecular Structural
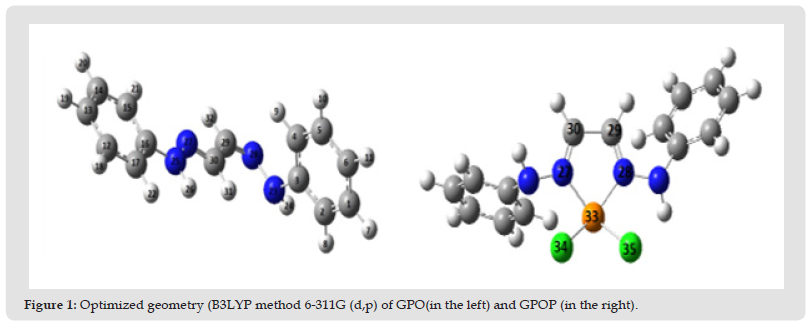
The optimized geometry (opt-freq) using (B3LYP method 6-311G (d,p) of GPO and GPOP were calculated. The numbers of atoms were defined in (Figure 1).
Figure 5 Figure 1: Optimized geometry (B3LYP method 6-311G (d,p) of GPO(in the left) and GPOP (in the right).
Spectroscopic Characterization of GPO and GPOP
The FT-IR spectra of the GPO and GPOP are listed in the experimental section (Figures 2 & 3). The vibrational band ν(N-H) shifted from 3304 cm-1 in the free ligand to 3275 cm-1 in the complex, due to the different spatial orientation of this group in the complex. The vibrational band ν(C-H) shifted from 3032 cm-1 in the free ligand to 3035 cm-1 in the complex. The spectrum of the complex is higher compared to the spectrum of free ligands because of the presence of coordinated ligands. The FT-IR spectra of ligand showed absorption bands at 1597 cm-1 , 1564 cm-1, 1504 cm-1, 1456 cm-1 in GPO and 1593 cm-1, 1564 cm-1, 1496 cm-1 in GPOP which were assigned to the ν(C=N) and ν(C=C) vibrations, respectively. These absorption bands in the free ligand are stronger than in the complex.
Spectroscopic 1H-NMR Characterization of GPO and GPOP
The 1H-NMR spectra of the ligand and complex in the DMSO-d6 solution are listed in the experimental section (Figures 4 & 5). The 1H-NMR spectrum of the ligand and complex showed a broad singlet at 10.37 and 10.38 ppm for the –NH group, respectively. This difference is due to the coordination of nitrogen atoms to palladium and the formation of Pd-N bonds.
Frontier Molecular Orbital Analysis
Investigation of the HOMO and the LUMO is important in a molecule as a ligand. The LUMO energy explains the ability to accept an electron and the HOMO energy is related to the ability to donate an electron. Both the HOMO and the LUMO play a significant role in the electrical properties and chemical activities in the compound [33] The HOMO and LUMO orbital energy are important parameters to predict the chemical properties of the title compound [34,35]. The HOMO and LUMO orbital energy of GPO are calculated at the B3LYP method 6-311+g (p,d) basis set. The energy values of the GPO are, EHOMO = -5.582 and ELUMO = -1.994 eV, respectively. The energy difference between the HOMO and LUMO of the GPO is 3.588 eV. The energy of HOMO and LUMO orbitals of the GPO are negative that these compounds are stable and do not decompose spontaneously into their elements. According to Parr, et al. [36]. The molecule with a small energy gap is more polarization properties, has low kinetic stability, and is in general called a soft molecule. These molecules can be explained as the resistance toward the deformation of electron clouds and the polarization of chemical systems during the chemical process. Chemical softness is a useful concept for predicting the behavior of chemical systems and is related to the stability and low reactivity of a chemical system.
The chemical hardness is a useful concept for predicting the behavior of chemical systems and is related to the stability of a chemical system by using HOMO and LUMO orbital energies, the ionization energy and electron affinity of the ligand and its complex can be calculated as: I = -EHOMO = 5.582 eV and A = -ELUMO = 1.994 eV, respectively. The global hardness η and chemical potential μ of the ligand and its complex are given by using the relation η = (I - A)/2 = 1.794 eV, μ = -(I + A)/2= -3.788 eV and Electrophilicity index (ω) = μ2/2η = 3.99 eV, respectively. The calculated value describes the catalytic and biological activity of the title compound (GPO). The atomic orbital components of the frontier molecular orbital are shown in (Figure 6).
Molecular Electrostatic Potential
Molecular electrostatic potential (MEP) is an important tool to predict the electrophilic and nucleophilic attacks for biological interactions [37,38]. The MEP of the title compound was optimized geometry using the B3LYP method 6-311+g (p,d) was calculated. As can be observed in (Figure 7). The different colors in this plot are identified as different values of the electrostatic potential. Red < orange < yellow < green < blue. Blue indicates the strongest attraction and red indicates the strongest repulsion. Regions of negative potential are with the lone pair of electronegative atoms. The negative electrostatic potential relates to an attraction of the proton by electron density in the molecule (shades of red), while the positive electrostatic potential relates to the repulsion of the proton by the atomic nuclei (shades of blue). The positive area is located around phenyl groups. These areas are having positive potential. The negative area is related to nitrogen atoms. These areas have a negative potential. The residuals species are surrounded by zero potential. The nitrogen of atoms with free-electron is as anions coordination to palladium and the formation of the complex.
Heck Reactions in the Presence of GPOP as Catalyst
The Heck reactions were investigated at two different temperatures (80 °C, and 120 °C), with DMF as a solvent and the amount of catalyst (0.08 gr, 0.2 mol) in the presence of Na2CO3 as a base for 24 h. With this new class palladium (II) catalyst, we investigated applying it to the construction of the C-C bond via cross-coupling reactions. The results of Heck’s reactions are given in (Table 1).
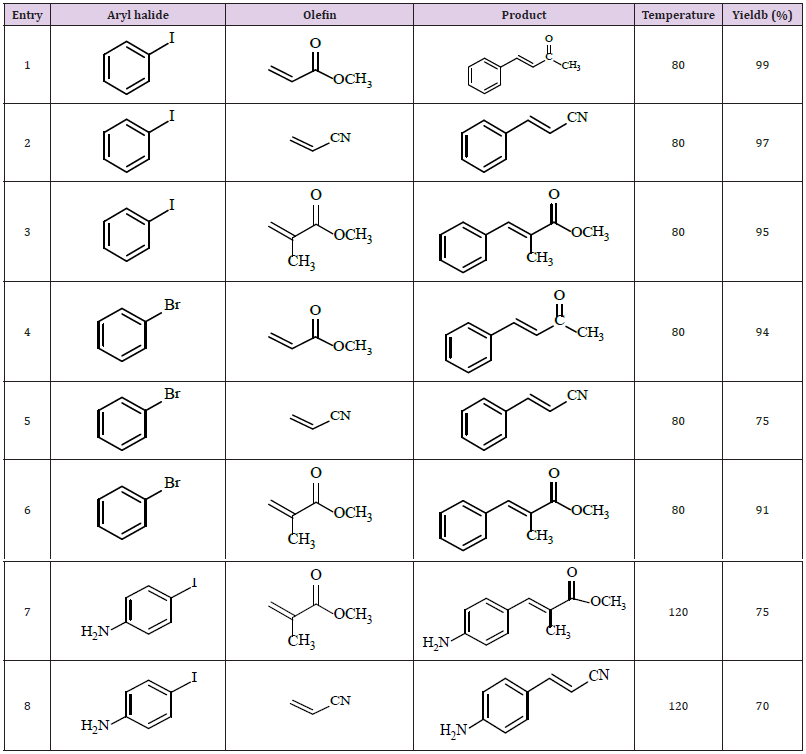
Table 1: Heck coupling of aryl halides with methyl and methyl methacrylate and acrylonitrile catalyzed by GPOP.
Note:
a. Reaction conditions: aryl halide (10 mmol), olefin (30 mmol), Na2CO3 (14 mmol), DMF (100 ml), catalyst (0.2 mol), for 24 h, under air.
b. Isolated yields.
Synthesis of the Methyl Cinnamate: Iodobenzene 1mL (10 mmol), methyl acrylate 2.7 mL (30 mmol) and, using DMF as a solvent (100 mL), Then Na2CO3 as base 1.5 gr (14 mmol) and catalyst 0.08 gr (0.2mol) were added. The stirring solution was refluxed at 80 °C for 24 h. (A pale-yellow solid; m.p. 34°C). IR(KBr): 3062(m), 2942(m), 2843(m), 1718(s), 1638(s), 1450(m), 1440(m), 1202(m), 1170(m). 1H NMR (CDCl3) 3.83(s, 3H), 6.47(CH, vinyl), 7.36(CH, phenyl), 7.55(CH, phenyl), 7.73(CH, vinyl). (S1).
Synthesis of the Methyl α- Methyl Cinnamate: Iodobenzene 1 mL (10 mmol), methyl methacrylate 3.2 mL (30 mmol) and, using DMF as a solvent (100 mL), Then Na2CO3 as base1.5 gr (14 mmol) and catalyst 0.08 gr (0.2 mol) were added. The stirring solution was refluxed at 80 °C for 24 h. (A pale-yellow solid; m.p. 35 °C). IR(KBr): 3030(m), 2941(m), 2951(m), 1716(s), 1633(s), 1494(m), 1440(m), 1303(m), 1116(m). 1H-NMR (CDCl3) 2.21(s, 2H), 3.85(s, 3H), 6.48(CH, vinyl), 7.41(CH, phenyl), 7.56(CH, phenyl), 8.01(CH, vinyl) (S1).
Synthesis Of 4- Amino Methyl Cinnamate: 4-Chloro aniline 1.27 gr (10 mmol), methyl methacrylate 2.7 mL (30 mmol) and, using DMF as a solvent (100 mL), Then Na2CO3 as base 1.5 gr (14 mmol) and catalyst 0.08 gr(0.2mol) were added. The stirring solution was refluxed at 120 °C for 24 h. (A pale-yellow solid; m.p. 35 °C). IR(KBr): 3453(m), 3392(m), 2953(m), 2186(m), 1724(s), 1602(s), 1502(s), 1438(m), 1303(m), 1118(m). 1H-NMR (CDCl3) 3.61(3H), 3.75(2H, NH), 5.85(CH, vinyl), 6.66 (CH, phenyl), 7.17(CH, phenyl), 7.28(CH, vinyl) (S1).
Synthesis of 3-(4-Amino Phenyl) Acrylonitril: 4-Chloro aniline 1.27 gr (10 mmol), acrylonitrile 1.96 mL (30 mmol) and, using DMF as a solvent (100 mL), Then Na2CO3 as base 1.5 gr (14 mmol) and catalyst 0.08 gr (0.2mol) were added. The stirring solution was refluxed at 120 °C for 24 h. (A pale-yellow solid; m.p. 35 °C). IR(KBr): 3412(m), 3221(m), 2975(m), 2186(m), 1494(m), 1410(m), 1116(m). 1H-NMR (CDCl3) 3.75(2H, NH), 5.92(CH, vinyl), 6.61 (CH, phenyl), 7.11(CH, phenyl), 7.27(CH, vinyl) (S1).
Molecular Docking Studies
We decided to perform the molecular docking simulation of the title compound against the 3D crystal structure of DNA, Escherichia coli, and HSA obtained from Protein Data Bank (PDB ID: 423D, 2JVU, 1AO6) respectively. Molecular docking is a significant investigation to understand the ligand-receptor interactions. The osazone complex was prepared for docking by B3LYP method 6-311g (p,d) basis set. These crystals were prepared for the docking system. Flexible ligand docking was carried out by Auto Dock 4.2.5.1 molecular docking program using the implemented empirical free energy function and the Lamarckian Genetic Algorithm [34]. We decided to show this compound has an antimicrobial property in the human body. The Cu complex as a ligand was prepared for docking by the B3LYP method 6-311g (2p,2d) basis set. In the first step, the docking of this complex with 423D, 2JVU, 1AO6, a blind docking with 126 lattice points along X, Y, and Z axes was performed to find the binding site of the complex on these crystals with a grid point spacing of 0.375 Å, to allow the complex to rotate freely. In the next step, the second docking was performed using a cubic box with 60×60×60 Å dimensions. Among the docked conformations, the best-scored conformation predicted by the Auto Dock scoring function was visualized for complex-HSA, complex-DNA, and complex- 2JVU interactions in Auto Dock software.
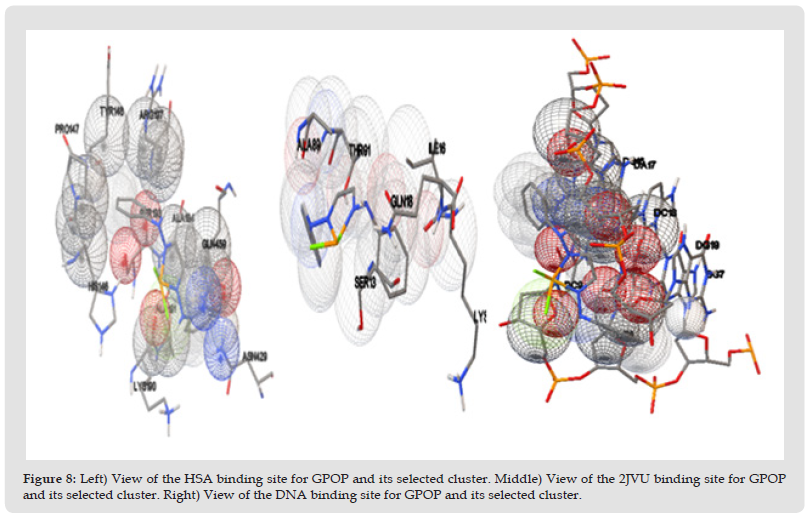
The resulting docking for the obtained molecular docking in which the osazone complex binds into HSA, DNA, and 2JVU, as a receptor is illustrated in (Figures 8-16). There are hydrophobic contacts between the osazone complex with HSA (PRO147, TYR148, SER193, ARG197, SER193, GLN459, ASN429, LYS196, HIS146, ALA191, ALA194) and 2JVU (GLN18, ALA89, THR91, SER13, LYS17, ILE13), and DNA (DA17, DG16, DC9, DC18, DG19, DT8, DG7) respectively. The binding free energies (ΔG°) of -6.57, 5.28, and -6.43 kcal mol-1 were predicted for HSA, 2JVU, and DNA in the best conformation of the complex.
Figure 12 Figure 8: Left) View of the HSA binding site for GPOP and its selected cluster. Middle) View of the 2JVU binding site for GPOP and its selected cluster. Right) View of the DNA binding site for GPOP and its selected cluster.
In order to show the application of heterogeneous catalysts, palladium-catalyzed coupling reactions have been found in organic synthesis. The Heck coupling reactions represent a powerful method for the C-C bond formation. We introduced palladium complex (GPOP) as the catalysts in the Heck reaction. The Heck reaction was performed by using iodobenzene, bromobenzene, and 4-chloroaniline with methyl acrylate, methyl methacrylate, and acrylonitrile as substrates in the presence of Na2CO3 as a base, and DMF as solvent at a certain temperature for 24h. HOMO-LUMO analysis and Molecular Electrostatic Potential studies of the GPO have been investigated using the DFT method. Molecular electrostatic potential and frontier molecular orbital analysis indicated the GPO can be applied as a ligand to form GPOP. Docking simulation of the GPOP with DNA, 2JVU, and HSA suggests that GPOP can be used for the design and synthesis of new based-drug materials.
We are grateful for the financial support from University of Lorestan.


