Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mehdi Rostamizadeh1*, Alireza elmieh2, Farhad rahmaninia3, Tayebe javan4 and Roya emamalizadeh5
Received:November 16, 2022; Published:December 07, 2022
*Corresponding author: Mehdi rostamizadeh, Assistant professor in exercise physiology, Department of physical education, Islamic Azad university of Ahar branch, Ahar, Iran
DOI: 10.26717/BJSTR.2022.47.007511
Objectives: Recent studies have shown that exercise by affecting bones causes
the release of hormones that are effective in regulating blood glucose, lipid profile and
preventing vascular calcification. The present study aims to Comparison effect of aerobic
and resistance exercises on Osteocalcin and Metabolic Profils in Overweight men.
Methods: Total of 33 overweight healthy men (BMI 29 = 67.96 ± 0.96 and age
33.2 ± 2.23) were randomly assigned into three groups: control (n = 11), aerobic (n =
11) and resistance exercise (n = 11) groups. The training program was conducted for
8 weeks, 3 sessions per week for the training groups. Body compositions parameters
(weight, body fat percent, BMI) were analysed, osteocalcin and blood biochemical parameters
were assessed from fasting blood samples before and after 8-week exercise
programme.
Results: Body fat, BMI and body weight reduction following exercise (P<0/05),
significantly increased serum Osteocalcin (P<0/05, baseline vs Post exercise) and
blood biochemical parameters significantly changes (P < 0/05). Increase in osteocalcin
had a negative correlation with changes in body composition, as well as HOMA-IR
and HDL, LDL not significantly (all P > 0/05).
Conclusion: Aerobic and Resistance exercis cause to changes in body weight,
body fat, BMI, as well as the increase level of osteocalcin and improvement of glucose
metabolism and lipid profiles. However, the difference between the two training
groups was not statistically significant.
Keywords: Osteocalcin; Body Composition; Metabolic Profiles; Lipid Profiles; Overweight
The World Health Organization has declared that [1] nearly 2 billion people are overweight and more than 500 billion worldwide are obese. The prevalence of overweight and obesity is related to the development of cardiovascular diseases, cancer, T2D, high blood pressure, dyslipidemia, and mental health disorders [2]. Different strategies have been applied in the management of obesity and the prescription of aerobic physical exercise has been very efficient for this purpose. There is already much evidence to support the notion that in overweight or obese individuals, the later physical activity is incorporated into the routine, the higher the metabolic cost, as numerous health-related variables worsen in short periods. Among the many benefits of aerobic physical exercise, are the reduction in adiposity and improvement in glucose metabolism [3,4] and the increase in exercise tolerance regardless of weight loss [5].
Exercise profoundly affects all tissues and organs: the more intense and greater volume is the activity, the greater is the response. Different kinds of exercise (e.g, endurance vs. resistance, aerobic vs. anaerobic, continuous vs. intermittent) differently affect the homeostasis and, hence, the adaptive response [6]. Adaptation to exercise contemplates the integration of primary (direct response) and secondary (response to soluble factors released by a third tissue) mechanical, endocrine, metabolic, and inflammatory responses each one proper of a different tissue. Then, these responses differ between acute and chronic exercise (training) since long-term adaptation implies changes in cell functions [7]. Recent studies have identified a novel mechanism by which cells involved in bone formation (osteoblasts) regulate glucose metabolism, modulating both b-cell insulin secretion and peripheral insulin resistance. These studies implicating that osteocalcin (OCN) is a novel bone-derived endocrine regulator of glucose metabolism [8]. Osteocalcin has been shown to enhance glucose and lipid metabolism, promote insulin and increase muscular mitochondria and pancreatic b-cell proliferation in animal models [9].
Importantly, OCN is known to increase with acute bouts of exercise, such as 4 × 4-min cycling at 95% maximum heart rate HIIT [10]. It directly promotes glucose and FFA uptake by skeletal muscle and establishes a positive feedback loop with IL-6, which also results in increased glucose and FFA utilization by skeletal muscle and the release of bioactive OCN by bone [11]. Metaanalyses of clinical studies showed inverse correlations between OCN levels and BMI [12], insulin resistance [13], body fat mass [14], and specifically, visceral fat mass, determined with radiologic imaging studies [15,16]. OCN promoted the survival and function of pancreatic β-cells and increased insulin secretion [17], while insulin itself induced the release of OCN [18]. It is thus possible that exercise, which stresses the bone via increased mechanical loading, would have an effect on markers of bone remodeling, including osteocalcin [19]. Reports of the effect of exercise on osteocalcin are contentious, with some researchers describing increases in total osteocalcin concentrations with acute exercise [20], while others reported lower, or unchanged osteocalcin concentrations shortly after exercise [21].
Recent studies in mice and human demonstrated the potential effect of skeleton in glucose and lipid homeostasis, which was regulated by OC [22]. Iki et al, investigated the association of the serum OC and glycemic status and insulin resistance in Japanese males’ population and demonstrated that serum OC was negatively correlated with FPG and HbA1C [23]. Wang et al, reported there was no relationship between OC and FPG [24]. Chin et al, demonstrated that most of the lipid parameters (LDL-TG) except for HDL had no significant association with osteocalcin levels [25]. Osteocalcin levels increase after weight loss in overweight adults [26]. Reinehr and Roth, reported a reduction in osteocalcin levels with increasing body weight and that substantial weight loss was associated with an increase in osteocalcin levels [27]. Obesity can be an independent risk factor for high blood triglycerides and HDL, LDL. Indeed, there is no doubt that obesity-related lipid disorders play an important role in atherosclerotic and cardiovascular diseases in obese people. However, since many obese people due to orthopedic, cardiopulmonary, and pulmonary disorders cannot participate in aerobic activity, several studies have shown that regular resistance exercises may be appropriate therapies [28].
In contrast to meal consumption, acute exercise transiently increases circulating levels of OC, and perhaps also tOC, which correlates with improved glycemic control and increased postexercise insulin sensitivity in humans [29,30]. It was reported that continuous moderate-intensity cycling exercise (CMIE) and high-intensity interval cycling exercise (HIIE) had little effect on the postprandial suppression of tOC and OC [31]. Levinger et al, reported that an increase of 6–14% in OCN on the human subjects after a single session of high-intensity aerobic exercise [29]. Also, Clemmensen et al, demonstrated that a 2.5-fold increase was reported in mice during and shortly after a single bout of aerobic exercise [30]. In general, controversial findings of the research that effects of different exercises on bone metabolism shown that several factors such as the type of exercise activity, age and sex may affect the response of bone metabolism indices to the exercises Put up [32,33].
Compelling evidence shows that aerobic exercise has an active effect on receptor affinity (adipose tissue, skeletal muscle and insulin receptors), thereby inducing insulin sensitivity and glucose homeostasis. Resistance exercise can enhance muscle strength, insulin sensitivity and muscle rehabilitation [34]. Regarding the importance of osteocalcin in regulating glucose metabolism and secretion of insulin from beta cells of the pancreas, and the contradiction in the findings of the studies of the effect of different sports activities in this field, it seems that finding a mechanism by which to increase the secretion of osteoclasin It is effective in preventing metabolic diseases. Therefore, the present study aimed to Comparison of the effects of aerobic and resistance exercises on bone turnover markers, glucose metabolism, Lipid profiles in overweight young men.
Study Design
This semi-experimental study compares aerobic and resistance exercise training in overweight mens. Between September 2019 and December 2019 in Ahar city, participants were recruited using social media platforms and community events. Following prescreening, participants were asked to come to the Cardiometabolic Exercise and Lifestyle Laboratory for additional screening and baseline testing. Overall, 56 youth were screened and deemed eligible for participation, and a total of 33 participants completed the study (Figure 1). All Participants provided written informed consent form before participating in this study. Study protocol was approved by the Ethics Committee of the University of Rasht branch. IRCT code: 20180226038876N1.
Inclusion Criteria for Participants
for this study Inclusion criteria includes age between 28-35, male gender, non-athletic, nonphysical disorders.
Exclusion Criteria for Participants
for this study Inclusion criteria includes: womens, smokers, cardiometabolic disease, athletics and high bmi (>30).
Participants
Thirty-three overweight males (mean age: 32.2 ±2.25 years; BMI: 28.85 ±0.96 kg/m2) who met the criteria for overweight for adult Asians established by World Health Organization (WHO) were recruited. All subjects were absence of systemic diseases, infections and physical disabilities, no smoking and no athlete. The subjects were randomly assigned either to the control (C, n = 11) or the aerobic exercise (A Ex, n = 11) group and resistance exercise (R Ex, n = 11) groups.
Training Programs
Aerobic training performed (aerobic exercise training 3 days per week for a total of 8 weeks. Initially, participants performed cycling or walking sessions for 30 min/session at a relatively low intensity. As their exercise tolerance improved, all participants increased the exercise intensity (65% in weeks 1–2, 70% in weeks 3–5, 75% in weeks 6–8) and duration (40 min/session in 2 first weeks, 50 min/session in weeks 3–5, and 60 min/session in weeks 6–8) [35]. Subjects in resistance exercise group, participated in 8-week supervised exercise training programme of three sessions per week (3 days/wk, 3 sets/day, 8–12 repetitions/set). Initially, 5 to 10 minutes of warm-up followed by main exercises (chest compression, armpit pull, head bolt from behind, foot press, forehead and back thigh), with intensity 50-75%, started a maximum repetition and resting time of 60-90 seconds, and every four weeks a new maximum repetition of the subjects was calculated, and the weight values were adjusted again [36]. Due to the non-athletic nature of the subjects, the Brazilian equation was used to determine a maximum repetition in resistance exercises: 1RM = Weight value/ 1.0278 - 0.0278×(repetitions) the subjects in Control group maintained normal lives.
Anthropometric Evaluation
Body weight were measured by Digital Scale (sahand; co. iran, the nearest 0/1 kg) and height were measured by Seca height device (japan tecnology, 0/1 cm) and body mass index (BMI) was calculated as weight/height2 (kg/m2).
Biochemical Analyses
Blood samples were collected from all participants after 8 h of fasting between 8:00 and 10:00 a.m. to minimize hormonal rhythmicity at the start of the study and 2 days after the termination of the study. Samples were immediately centrifuged at 3000 g for 10 min at 4 °C and serum and plasma samples were stored at -80 °C until analysis.
Metabolic Profile
Metabolic profile consisted of Fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL). Lipid and glucose profiles were obtained using the Autoanalyzer devise (Technicon, RA1000, USA) Pars Azmoon kits (Karaj, Iran). Serum insulin concentrations were determined in duplicate using ELISA (ALPCO, Salem, NH, USA). HOMA-IR was calculated according to the following formula: fasting serum insulin (μU/ml) × fasting plasma glucose (mmol/l)/22.5 [37]. HbA1C were Quantified access shield kits (England) by using a devise (LabonaCheck A1C & Cera-Stat 2000). The serum levels of OC were determined by Human OT/BGP (osteocalcin) ELISA Kit (Hangzhou Eastbiopharm co. LTD; CHINA) with sensitivity of 0.026 ng / ml by sandwich ELISA was measured and using the instructions given in the brochure kit.
Nutrition
Calorie intake was assessed using a 3-day food record and a 24-h recall interview conducted at the beginning and end of the training period. Dietary intakes recorded from the 3-day records and 24-h recalls were analyzed for calorie and macronutrient content using Food Processor Nutrition Analysis Software (Version 7.1, 1996, ESHA Research, Salem, OR), which provides access to information on over 15,000 food items with data for 105 nutrient components. Confirmation of nonsignificant variability between the two measures permitted us to combine the two measures and calculate a mean energy intake over 4 discrete days at each time point.
Statistical Analyses
Data were presented as mean ± SD. We used paired t tests to assess differences in traditional biomarkers of between baseline and after 8 weeks of exercises, for the entire cohort. Comparisons of the variables among the groups were made using ANOVA, LSD posthoc analyses were used to identify any timepoint differences in OC concentrations. For variables not exhibiting normal distribution, Kolmogrov - Smirnov test was performed. Data management and statistical analyses were performed using SPSS version 23. A p ≤ 0.05 was considered significant.
All subjects completed the 8‐week aerobic and resistance exercise training program. The average frequency of exercise training was 3.1 ± 0.3 days/week in supervised sessions. After the training VO2max were significantly Increased (P < 0.05). As per the study design, there were no significant differences in age, weight, BMI and other measurement factors between all groups. As reported, body weight decreased similarly in the aerobic group (−4.94 ± 0.58 kg [6% decrease], resistance group (−1.35 ± 1.00 kg [3% decrease]) but not in the control group (−0.68 ± .12 kg [<1% decrease]) (Figure 2). The changes in OC, TG, INS, HDL, HbA1C between the resistance and aerobic groups were not different (P> 0.05) (Figure 3) But significant different showed in LDL and COL between all groups (P< 0.05). Serum OC concentrations increased more in the aerobic group (2.92 ± 0.37μg/L [11% increase] than in the resistance group (1.34± 0.95 μg/L [5% increase], whereas they did not significantly change in the control group (−0.55± 0.017 μg/L [1% decrease]) (Figure 4).
Table 1. Line bar associations among baseline OCs and the measures of anthropometry and blood biochemical parameters.
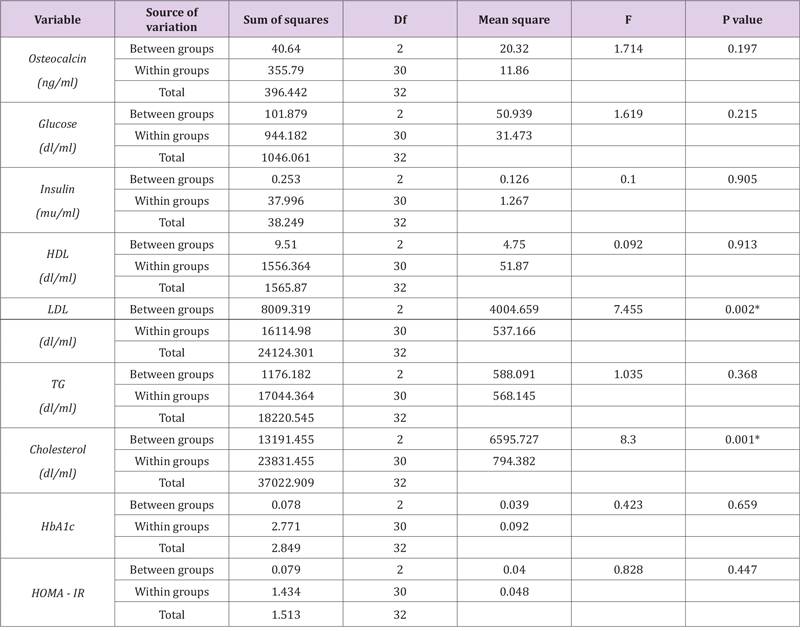
Body composition and Biochemical parameters were improved in both Two Exercise trainings group (RT and AT). Specifically, weight, BMI, total fat % (P < 0/05) were significantly decreased in both two Ex Group (RT and AT). Also, Ex groups showed decreased Insulin, HOMA-IR, LDL, HDL (P < 0/05). There were no significant differences in fasting plasma glucose, TG, HbA1c on resistance Ex group. Considering the difference in mean on comparison between groups, Results showed that the effect of aerobic training was more than resistance training. The correlations between changes in body composition parameters and circulating levels of OC’s were evaluated. Pearson correlate showed that changes in BMI, total fat% no significantly correlated with changes in total OC level in all subjects (P > 0/05). Also, Pearson correlate showed that changes in biochemical parameters not significantly correlated with changes in total OC level in all subjects (P > 0/05). And there is only a positive correlation between osteocalcin and blood glucose, which is statistically significant (r=-0/373, p=0/025) (Table 1).
The results of the current study indicate that resistance and aerobic exercise, resulted in attenuated weight loss. Compared with the significant increase in bone turnover markers in the aerobic and resistance exercise group, markers of bone turnover were not significantly increased in control group. Also, we demonstrated that 8-week exercise training lowers body fat mass, weight, BMI and changed biochemical parameters level and improves Insulin resistance in overweight young males. Although the effect of aerobic training on these factors was higher in comparison with the two experimental groups, these differences were not statistically significant.
Physical activity is recognised as one of the most effective lifestyle strategies to maintain bone health and metabolic function during lifes [38-40]. This effect of exercise has been shown, at least in part, to be mediated/associated with the exercise effect on bone metabolism, including its effect on OC [41,42]. Recently, increasing attention has been directed toward the role of OC in modulating glucose and lipid metabolism. Lee et al. showed that OC is a bone messenger affecting both adipocytes and insulin-producing β-cells. It increases β-cells proliferation, insulin secretion, insulin sensitivity, adiponectin expression and energy expenditure by upregulating the expression of adiponectin gene in adipocytes [43].
In our study, serum concentrations of OC increased after 8 week aerobic and resistance exercise. Ghorbanian et al, demonstrated that 10-week aerobic exercise in obese women leads to significant increase in osteocalcin levels, decrease in lipid profile and improvement in blood glucose [44]. Also, Atashak et al, reported that 10 weeks of resistance training leads to a significant increase in osteocalcin levels, decrease in lipid profile, decrease in blood glucose and improvement body composition in overweight mens [28]. In line with the findings of our research, they have reported that various exercises have reduced of LDL, TG, cholesterol, insulin and glucose Levels, and decrease BMI, increased levels of Osteocalcin, HDL, HbA1c and VO2max in different individuals with different sexes. Bone formation is a slow process, and we could hypothesise that to induce long-term changes in circulating levels of OC would perhaps require a longer intervention time frame [45]. This has been shown in a recent meta-analysis where regardless of the exercise modality, a training intervention greater than 8 weeks induces a significant increase in ucOC [46]. Impact and the modality of exercise training is also an important factor as bone mechanical properties are modified depending on workload where mechanical stress must reach a minimum level to promote structural changes in the bone [46].
Hiam et al, reported that no significant changes in oc levels follow up any type of exercise in men and womens [47]. Also, this finding Contrary to Findings of Nouri [48], inactive men, Attarzadeh [49], in obese women, bizheh [50] on middle-aged women whose reported non change or increase in biochemical markers and body composition. Furthermore, another objective of this study was the correlation between the level of osteocalcin and blood biochemical parameters in overweight men. Significant correlations were observed between changes in glucose and OC after exercise. This correlates, which is consistent with the findings of Kanazawa [51] on men, Iki [23] on young men, that have reported a correlation between baseline osteocalcin and blood glucose. Contrary to the findings of Wang [24] who reported a non-correlation between osteocalcin and blood glucose. Also, Chin et al, reported that most lipid (LDL-TG) parameters except HDL had a no Significant relationship with osteocalcin levels [25].
The reasons and mechanisms in comparing the effect of aerobic and resistance training on the levels of blood biochemical parameters and body composition, that is have energy consumption, during aerobic exercise is more than resistance activity, and that aerobic exercise is Done continuously, and Resistance activity is performed in the form of interval. In this way, fluid changes may be greater during aerobic exercise [40]. The contradiction in the results of various research can be attributed to differences in the type, severity, duration and repetition of activity as well as the degree of physical fitness and age of different individuals. Meanwhile, the intensity of exercise pressure is the main cause of the contradiction in the responses of the factors studied.
Concentrations of HDL, LDL as well as their plasma ratios are risk factors for heart disease and can also predict cardiovascular disease in the future [41]. Increasing HDL leads to preventing cholesterol deposition in the blood vessels, on the other hand, performing exercise activities, especially aerobic exercises, results in more fatty metabolism, resulting in more fat to supply energy. Research has shown that endurance activities increase HDL and guarantee the health of individuals [45]. Some studies have shown that aerobic exercise has a much greater effect on blood lipoproteins than resistance exercises. In a study by Farahingam in 2002, it was found an increase in each mg of HDL, of the reduction in 2 to 3 percent of cardiovascular disease. physical exercises is an appropriate remedialy for improving insulin resistance [46].
Long-term exercises can increase glucose transporters to muscle cells and insulin receptor substrates, as well as increase in muscle mass (more than 75% of glucose utilization due to insulininduced muscle tissue stimulation) cause response of body on Insulin and increases insulin sensitivity and preventing obesity and its subsequent complications. The role of aerobic exercises has been shown to increase insulin function by reducing intracellular TG accumulation and increasing the oxidation of fatty acids [47].
Exercise unleashes a complex network of endocrine interactions in which circulating factors, released in response to exercise, interplay through inter-organ crosstalk and physiologic changes. Exercise influences a favorable organokine profile that, per se, mediates many of its beneficial health effects. In summary, acute exercise bouts appear to modulate the release of myokines, hepatokines, osteokines, and immune cytokines, while chronic exercise training correlates with changes in basal circulating adipokines and immune cytokines, possibly in association with weight loss itself. In conjunction, organokines act in an orchestrated manner to modulate systemic metabolic processes. Their concentrations and effects change in response to the intensity, duration, and frequency of exercise to directly or indirectly control WAT reserves.
one of Beneficial organokines induced by exercise is a osteocalcin. it is plausible that, in addition to protecting against the development of obesity, exercise, when performed at a sufficient frequency, intensity, and duration, is a powerful tool for dissipating excess visceral fat mass that is causally involved in the pathogenesis of chronic Cardiometabolic diseases [48-50]. It seems that 8 weeks of aerobic exercises has been able to minimize Oxidative stress, cause of optimizing change on osteocalcin and fats and glucose metabolism, as well as improving respiratory and cardiovascular capacity in overweight men. Lipid disorders, like elevated of triglyceride and cholesterol levels, and lowering HDL levels are one of the most common lipid disorders associated weight gain, which is the main causes of increased incidence in cardiovascular disease and with exercise and changes in body composition such as body fat % and WHR and BMI in overweight individuals Leading to reduce the risk of metabolic diseases.
Important areas to address in future research include the translational potential of the preclinical evidence, the identification of relevant effects of exercise-inducible factors from a clinical perspective, and, finally, the inter-individual variability of the physiologic response to exercise interventions, especially in obese populations. Specifically, [51] considering that groups of exerciseinducible factors have been mostly studied independently, future interventional clinical studies with large sample sizes should address a comprehensive panel to evaluate the status of all groups of organokines, ideally at the tissue level (when feasible) and in circulation. This type of research ought to be carried out before and after predetermined time intervals following acute exercise bouts and chronic training regimens of different modalities, durations, and intensities, both in healthy controls and patients with obesity and associated metabolic diseases. Studies in humans are important, given that results could differ from those obtained in preclinical models, possibly aiding in the identification of the most viable therapeutic targets [52].
The results of this study showed that both aerobic and resistance training significantly increased the serum levels of blood biochemical indices and body composition in overweight young men. In spite of the fact that, in comparing the meanings show changes in aerobic training group greater effect more than the resistance training in changes on levels of factors studied. But these differences were not statistically significant. Thus, in summary, aerobic and resistance training, both with increasing mechanical load on bone mass, causes changes in energy metabolism and body weight and can be an important factor in increasing bone mass and weight loss in overweight and obese people.
Thanks, and appreciation of the efforts of the supervisors and consultants and all the loved ones who have tried in this field.


