Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ali Torabi1*, Nuray bozkurt2, Süleyman Nergiz3, Ebru Marzioğlu Özdemir4, Nadir Koçak5 and Tülün Çora6
Received: November 24, 2022; Published: December 07, 2022
*Corresponding author: Ali Torabi, Medical Genetics Research Associate, University of Selcuk, Faculty of Medicine, Department of Genetic, Konya, Türky
DOI: 10.26717/BJSTR.2022.47.007507
In normal platelet count, Differential diagnoses of platelet-type bleeding diathesis are complicated. The reasons can be the shortfall of a clue in routine laboratory workups or the milder clinical picture that results in fewer clinic visits. Here we report a 39-year-old female who experienced progressive hematuria episodes following her in-vitro fertilisation (IVF) procedure that started alongside with administration of 150 mg daily aspirin orally. The patient’s lab work showed no reason for hematuria. P-LCR, PDW, and MPV were slightly increased. The patient’s and her family’s clinical history did not direct any clear clinical diagnostic clues. The platelet function test by the aggregometry study was also ambiguous for any net diagnosis. The list of the phenotype-related genetic pathogenic variants was revealed following Clinical exome sequencing and SNP microarray-based genetic variant analysis. As a result, three variants for GPVI, three for ITGA2(GPIa), and one for ITGB3(GPIIIa) genes were unveiled. The mentioned genes are all responsible for the expression of membrane glycoproteins that play a role in collagen-induced platelet adhesion, activation, and platelet-platelet interaction. On the other hand, GPVI’s relation with platelet size is also disclosed in the literature. Although all of the found variants’ clinical evidence statuses are benign, this can be because of the lacking case-control association studies. Pharmacogenetic data analysis for aspirin correspondingly showed the compound susceptibility toward increasing bleeding time (ITGB3 – rs5918; F13A1 – rs5985) and sensitivity to aspirin (LTC4S – rs730012). We offer that functional pathway synergism can empower the variant analysis significance in a single variant’s clinical evidence power deficiency. The additional pharmacogenetic analysis is recommended to announce possible medicine susceptibilities in suspicious patients.
Keywords: Bleeding Diathesis; Platelet; Clinical Exome Sequencing; Genetic; Microarray; Global Screening Array; Pharmacogenetic; Personalized Medicine; Genetic Variant Analysis
Abbreviations CES: Clinical Exome Sequencing; IVF: In-Vitro Fertilisation (IVF); P-LCR: Platelet Large Cell Ratio; PDW: Platelet Distribution Width; MPV: Mean Platelet Volume; PT: Prothrombin Time; INR: International Normalized Ratio; PTT: Partial Thromboplastin Time; PBS: Peripheral Blood Smear; ADP: Adenosine Diphosphate; VWF: Von Willebrand Factor; CSV: Comma-Separated Values; VCF: Variant Call Format; RT-PCR: Real- Time Polymerase Chain Reaction; FTP: The File Transfer Protocol; dbSNP: The Single Nucleotide Polymorphism Database; ITGB3: Integrin Beta 3; ITGA2: Integrin Beta 2; GPVI: Glycoprotein 6
Platelet function disorder in the presence of normal platelet count has been reported to lead to variable clinical phenotypes [1]. Differential diagnosis of these bleeding diathesis types has been much more complex than thrombocytopenic bleeding diathesis conditions. The reason can be the absence or shortfall of a clue in routine laboratory workups such as biochemical blood test results or peripheric blood smear studies. Another reason can be the milder clinical Picture of patients with these conditions that leads to later clinical visits. Occasionally, notwithstanding starting of clinical investigations, no diagnosis will be revealed. Nowadays, genetic analysis can be the solution for early diagnosis and monitoring of these patients. But sometimes, lacking the referral clinical evidence to analyse the revealed genetic variants may cause the proper diagnosis to be missed. Here we report a 39 years old woman who had massive gross hematuria following an in vitro fertilisation (IVF) procedure. A synergism in target pathways helped us uncover the disease’s pathogenicity.
The patient was 39 years old married woman who had undergone an IVF procedure for an unknown cause of infertility. A day after the embryo transfer procedure, progressive hematuria was initiated with urinary hesitancy and suprapubic pain during urination. The patient was transferred to the hospital emergency after gross hematuria characterised by blood clots, mild hypotension, and lightheadedness. During emergency admission, the patient’s vital sign was stable, with 90/60 mmHg blood pressure. Despite the stable vital signs, intravenous hydration was started for the patient and bedside ultrasonography revealed a massive bladder hematoma. The rest of the pubic and abdomen cavities were evaluated as normal in ultrasonography. The patient had been catheterised by an appropriate urinary catheter, an initial workup among lab test were taken and moved to the ward after being monitored for a while in emergency service. Urinary bladder Irrigation was considered after a urology consult, which resulted in draining the blood clots that had been continued intermittently for the next two days of admission until achieving clear gross urine. The patient was discharged after a control pubic and abdomen ultrasonography and other lab workups. For this patient, ovarian stimulation of the IVF procedure was started alongside the oral administration of 150mg aspirin daily. In the beginning, embryos decided to be transferred on the 3rd day of fertilisation, but it was postponed to the 5th day during the unsuccessful cervical passage try due to the narrow cervical opening to prevent any risk of cervical damage.
The second try was done after two dosages of misoprostol 200mcg administration for cervical ripening. The 5th-day transfer procedure was done without side effects, and the patient was discharged without any disapproving conditions. The preliminary lab work toward bleeding diathesis of the patient during the hospitalisation did not show any reason for hematuria. The hemogram of the patient only showed increased P-LCR, PDW and MPV. PT, INR, and PTT were normal either. Later, a series of the beta-HCG test confirmed IVF procedure failure. Further workup was continued in an outpatient setting to evaluate any cause of possible bleeding diathesis. In the medical history investigation, the patient was a mild smoker (1.25 pack-year) started five years ago. No history of frequent bruising, gingival bleeding, menorrhagia, or any other sign of moderate to severe bleeding diathesis was detected. The only suspicious histories that it may associate with bleeding diathesis phenotype were a course of post-rhinoplasty surgery with mild but intermittent nasal bleeding that stopped at about a week post-surgery by the massive tamponade procedure, and the post-traumatic ecchymosis that had occasionally been happening after a moderate trauma that did not seem to be exceptional. The patient had a history of micro-prolactinoma, migraine headache, breast fibroadenoma, and laparoscopic cholecystectomy due to cholelithiasis. No family history of the bleeding diathesis-related condition was detected in family history. The physical examinations of the patient were reported normal during the polyclinic visits. The patient was followed by haematology, then medical genetics polyclinic for additional workup, where the peripheric blood smear test, platelet function tests, genetic-based susceptibility to bleeding diathesis for revealing possible pathogenic variants, and pharmacogenetic analysis were planned.
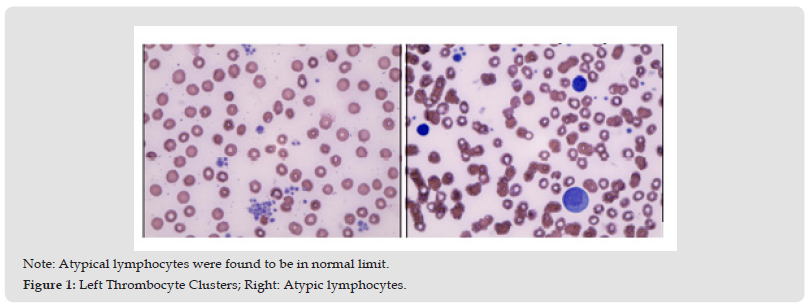
In the hemogram, except for P-LCR, PDW and MPV increase, no other findings were detected. PBS also revealed normal (Figure 1). The patient was checked up for platelet function analysis by aggregometry testing using ADP (0,01mmol/L and 0,02mmol/L), Collagen (0,005mg/mL and 0,010mg/mL), Epinephrin (0,005mmol/L and 0,010mg/mL), Ristocetin (0,6 and 1,5mg/mL) and arachidonic acid (1,5mg/ml) agonists. The aggregation response with ADP started with shape change. Inhibition and disaggregation were observed at the maximum response. Maximum response was slightly increased when the ADP dose was doubled, but the inhibition continued. Although minimal shape change was observed in the aggregation response with collagen, the lag phase was prolonged, and significant inhibition was observed in the maximum response. When the collagen dose was doubled, the expected shapeshift developed, and the maximum response increased. However, the maximum response was slightly inhibited. Secondary aggregation with epinephrine has developed, but the response was inhibited, and the maximum response increased while agonist concentration was increased. Aggregation responses were seen as normal by ristocetin at high (1.5mg/mL) and low (0.6mg/mL) doses. Aggregation response was very weak by arachidonic acid (Figure 2). The patient’s Ristocetin cofactor(VWF activity), VWF factor antigen (based on the patient’s related blood group – O-), and factor VIII were in the normal range. Previously Karyotype and Thrombophilia panels (by RT-PCR) were considered for the patients parallel with pre-IVF genetic control routine.
Figure 1 Note: Atypical lymphocytes were found to be in normal limit. Figure 1: Left Thrombocyte Clusters; Right: Atypic lymphocytes.
Results were normal for the karyotype and only showed MTHFR C677T HETEROZYGOUS mutation and 4G/5G genotype for the PAI-1 gene. For further genetic analysis, Clinical Exome Sequencing (CES) analysis and genotyping microarray were considered by DNBSEQ-G400TM sequencer (MCI tech., Co.) and Infinium™ Global Screening Array-24 v3.0 BeadChip - Illumina© respectively. These analyses were made for two different purposes, revealing clinical genetic pathogenesis and sensitivity to aspirin. Accordingly, the variant list in VCF (By using the Genomize-Seq® platform) and CSV (with Genome Studio application after quality control analysis) formats were revealed after CES and Genotyping Microarray, respectively. Further variant analysis was performed manually with Microsoft 365 Excel software. To do this, a tabular reference database was created after accessing the NCBI-Clinvar FTP server. This multicolumnar list consisted of the name of variants, genes, locations, dbSNP ID, clinical significance status, phenotype features, protein changes, related conditions, canonical SPDI and allele ID. This list was filtered using the “Platelet” keyword, and the most relevant variants were saved in CSV file format. This list was used as a database to be combined with the VCF and CSV files mentioned earlier (The patient’s CES and Microarray results). After merging all three files, the phenotypic filtering was applied again according to the clinic of nonthrombocytopenic bleeding diathesis. Accordingly, a CSV file with the final patient’s analysis result with the relevant explanation was created.
A similar methodology was utilised to perform the pharmacogenetic analysis of aspirin by using the PharmGKB.org database. All obtained gene pathways were analysed separately using the online databases of GENECARD.org and REACTOME.org. In the next step, the pathways were compared to assess the pathway homology and synergism. A total of 21 variants were found in the clinical pathogenesis study, with the following characteristics: 1- All were classified as benign clinical Interpretation, 2-According to the review status in the NCBI-Clinvar database, 17 variants had evidence from multiple studies without conflict, 2 were single enrollment, and two did not provide any statement of evidence, 3- 16 were in exonic regions, three in UTR3, and two in intronic regions, 4- It was reported that 11 of these variants were associated with multiple phenotypic or more severe clinical pictures that were not related to our case. All variants were checked for pathway homology. By excluding unrelated phenotypic features and the variants not involved in platelet functioning, a total of 7 variants were revealed by final filtration, as shown in (Table 1). Pharmacogenetic data analysis for aspirin (acetylsalicylic acid) showed compound susceptibility to increased bleeding time (ITGB3 – rs5918; F13A1 – rs5985) as well as sensitivity to aspirin (LTC4S – rs730012). With the pathway analysis of the relevant genes of the resulting variants and attention to the reciprocal pathway synergies between them, the collagen-platelet interaction can be presented as the pathogenesis point to explain this patient’s condition (Figures 3 & 4).
Figure 3 The Reciprocal Pathway Analysis done by Using REACTOME.org Online Platform. A Yellow Colour Resembles The Participation of The Patient’s Variants in The Particular Cascade.
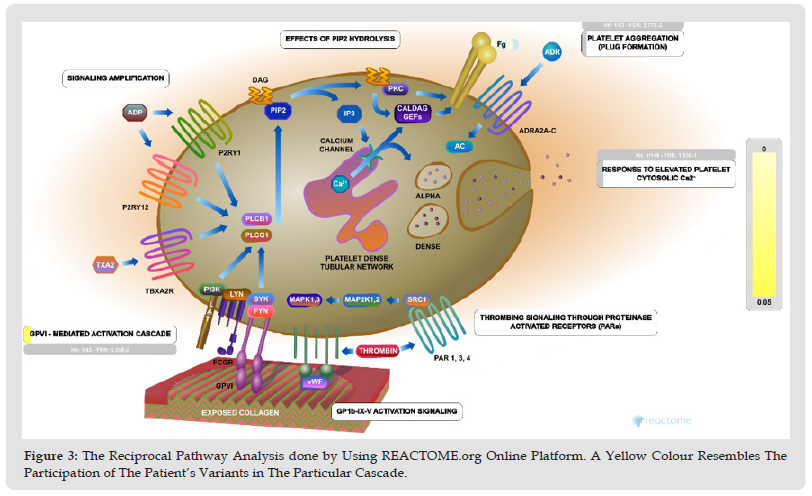
Figure 4 The Platelet Membrane Glycoproteins (Integrins beta3, alpha2 and glycoprotein 6) Are Shown to be the Main Reason for Pathogenesis. The Yellow Indicates the Extent of Pathogenic Variants Enrollment - From the REACTOME.org Online Platform.
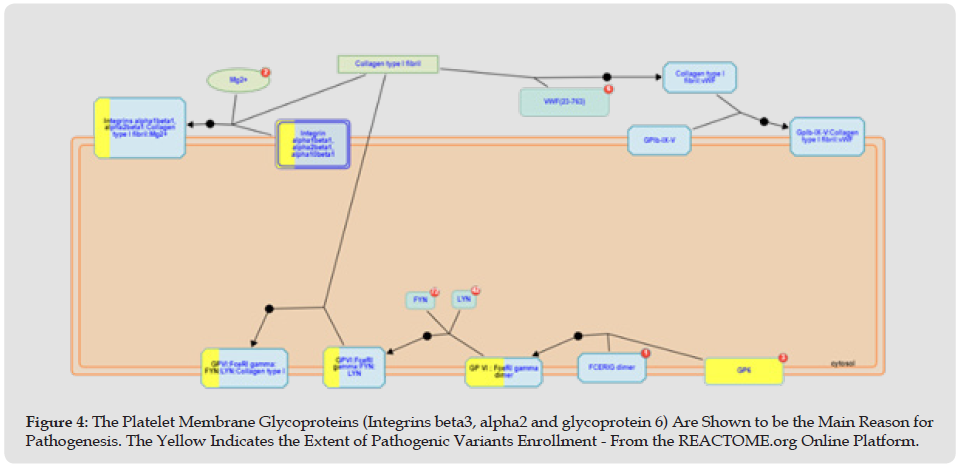
Platelet adhesion is the first step in platelet aggregation. Circulating platelets are captured and activated by the released collagen and vWF. Consequently, several collagen-binding proteins are expressed in platelets, including integrin alpha2 beta1, GPVI, and GPIV. Integrin alpha2 beta1 is the main platelet collagen receptor, and It requires Mg2+ to interact with collagen and may require mediated activation of the integrin alphaIIb beta3 for initiation. Binding to a collagen motif with the sequence Gly-Phe-Hyp-Gly-Glu-Arg occurs via the alpha2 subunit I domain. The binding of collagen to alpha2 beta1 produces intracellular signals that contribute to platelet activation. These facilitate the binding of the low-affinity collagen receptor GPVI, an essential receptor involved in collagen-induced platelet activation. The GPVI receptor is a complex of the GPVI protein with an Fc epsilon R1 gamma dimer. Src family kinases Fyn and Lyn constitutively associate with the GPVI: FceRIgamma complex in platelets and initiate platelet activation via phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM) in FceRI gamma, leading to binding and activation of the tyrosine kinase Syk. The influx of Syk leads to platelet activation via several adapter molecules and effectors. The vWF protein is a polymeric structure of variable size. It is secreted by the endothelium in two directions, basolaterally and into the bloodstream. Shear-induced aggregation is achieved when vWF binds via the A1 domain to GPIb (part of GPIb-IX-V) and the A3 domain, which mediates collagen binding to the inferior endothelium.
The interaction between vWF and GPIb is regulated by shear force; An increase in shear stress results in a corresponding rise in vWF’s affinity for GPIb [2-4]. Briefly, it can be concluded that the homologous genetic variation in the platelet-collagen interaction pathway facilitated by integrin alpha2 and beta 3 expressions as part of collagen binding proteins that induce the collagen-based platelet activation by GPVI is the mainstay of the patient’s bleeding diathesis. In addition, we believe this variant homology may explain the patient’s susceptibility to postoperative (collagen stress) bleeding diathesis. Indeed, the prolonged lag phase and maximum response by doubling the collagen dosage in the aggregometry test can disclose this kind of collagen-based platelet malfunction [2,5]. Various reports of variants in the literature have located the biological hotspot of bleeding diathesis pathogenesis in patients with normal platelet counts [1]. Among these variants, similar clinical and aggregometry pictures to our patients belong to the GPVI gene (which we have in our patient as well) and TBXA2R gene (Only one heterozygous variant was detected that was reported benign in Clinvar-NCBI and excluded in our filtering phase due to the absence of any clinical condition reported- TBXA2R: chr19:3595923 G>A). On the other hand, the biallelic ITGA2B/ITGB3 variant is associated with Glanzmann thrombasthenia in literature, which expresses more severe bleeding phenotypes and completely different aggregometry results (generally absence of aggregation to all agonists).
Even though GPVI variants were reported to have minor bleeding severities, they have an absence (not mildly reduced as in our case) of responses to collagen, convulxin and collagen-related peptide. Although the isolated absence of response to arachidonic acid agonist is more seen in TBXA2 receptor variants, we detected this response in our aggregometry test. However, the absence of response to arachidonic acid seems to be shared in different variants associated with bleeding diathesis alongside normal platelet counts [5]. The literature has already unveiled the relativity of the GPVI expression and platelet size [6]. This may also explain our patient’s mild increase in PDW, P-LCR and MPV. In summary, here we reported a patient with undiagnosed bleeding diathesis with a normal platelet count who suffered from the severe bleeding course accelerated by a 150mg daily dosage of aspirin with a history of a mild post-surgical tendency to bleeding. Susceptibility to aspirin and collagen-induced platelet activation pathway pathology is revealed to be a cause for the patient’s clinical picture. We want to conclude that, due to the benign interpretation status of the clinical classification of the unveiled variants, all or some still can be the pathogenic targets of our analysis, and it may be missed in the report because of case-control association studies deficit that withholds the clinical interpretation status. This case report can reveal the importance of pathway-targeted genetic variant analysis for early diagnosis and prevention of disease sequelae, especially in the absence of powerful clinical evidence data. In this approach, reciprocal pathways must be uncovered and analysed according to genes (variants) biological functions. An additional pharmacogenetic analysis of the drug in use by the patient, especially if that particular drug targets the same pathogenic pathway, brings considerable advantages in the clinical follow-up and prognosis of patients.
Here we also want to thank Dr. Metin Bağcı from the haematology polyclinic of Selçuk university, medical faculty, Konya, Ankara, because of his valuable consultations on this case.
All the authors confirm that they have no conflict of interest.


