Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gilberto Cruz Arteaga1, Vania Alejandra Valenzuela Rodríguez8, Alma Italia Guerrero Martínez2, José Antonio Zamudio González3, Guadalupe Santana Santiago4, Macedonia Guadalupe Moreno Tovar4, Rebeca Cortés Chamorro7, Janet Fabiola Perez Medina4, Dennice Cebreros Santiago4, Beatriz Cruz Arteaga7, Gloria Garnica Resendiz4, Monica Adriana Pineda Gutierrez4, Gisela Mata Cruz4, Olivia Guadalupe Villanueva Martinez4, Mariana Irais Guzman Carrera6, Arturo Andrade Sanchez5, Linet Nava Ramirez4, Jorge Ramón Moran Rubio4, Emilio Ramirez Medina4, Augusto de Jesus Sanchez Arriola4 and Miguel Alfredo Zurita Muñoz4
Received: November 03, 2022; Published: November 10, 2022
*Corresponding author: Gilberto Cruz Arteaga, Family Medicine Specialist, Family Medicine Unit No. 20, Calzada Vallejo No. 675, Col. Nueva Vallejo, CP 07750, Gustavo A. Madero Delegation, Mexico City, Mexico
DOI: 10.26717/BJSTR.2022.47.007448
The COVID-19 disease is caused by SARS-COV2, Mexico occupied one of the first places of mortality between January-August 2020 in Latin America, increasing the risk of mortality from comorbidities such as hypertension, diabetes and obesity. The prevalence of mortality with treatments in COVID-19 has been variable, 23% with Rivaroxaban, with little evidence of mortality in the mild phase of COVID-19. A controlled clinical trial study in COVID-19 mild phase reported 93% efficacy when administering Rivaroxaban/Azithromycin/Ivermectin, considering mortality would help to know the behavior in patients with COVID-19 mild phase. The objective is to estimate mortality in patients diagnosed with COVID-19 after a comparative treatment of early intervention in beneficiaries of the U.M.F 13 and U.M.F 20 of the I.M.S.S. Material and Methods: Non-experimental study, cross-sectional-retrospective-comparative-secondary data analysis, in 114 patients obtained from a database of a single-blind randomized clinical trial conducted and registered in clinicaltrials, the data was analyzed in S.P.S.S. V.21, evaluated by comparative group the status of living and dead patients with sociodemographic variables and number of COVID-19 days, followed up through frequency tables, using Chi-square test, survival analysis with Wilcoxon (Gehan) test, with p< 0.05 as statistically significant. Results: The percentage of mortality in COVID-19 patients was 1.7% in triple therapy vs. 0.9%, dual therapy, p= 0.778; The median cumulative survival of sick days in COVID-19 patients under the living and dead status was 4 days, with p=0.005.
In Mexico, mortality has reached national figures of 108,658 deaths so far from the COVID-19 Pandemic in the period between January-August 2020, representing the second cause of death at the national level, placing it in one of the first places of mortality in Latin America, with increased risk of incidence in people with comorbidities such as hypertension, diabetes and obesity [1,2]. That is why multiple initiatives have been developed that evaluate the capacity of various drugs in the treatment against SARS-CoV-2 and reduce morbidity and mortality, such as the use of ivermectin, azithromycin, low molecular weight heparin and steroids that are established in the Algorithm for the Attention of COVID-19 of the Mexican Institute of Social Security 2020 [3] . The use of ivermectin on the viral activity against SARS-CoV2 infection in Vero/hSLAM cells, it was observed that the treatment could cause a reduction of approximately 5000 times the viral load of SARS-CoV 2 at 48 hours of isolation, establishing the Ivermectin as a potential antiviral drug with reduction of disease progression [4]. The antiviral effects of azithromycin suggest the ability to induce pattern recognition receptors that stimulate genes and interferons, leading to reduced viral replication, as well as interfering with virus entry through the interaction of the host protein ACE2 and the protein viral spike [5]. The use of anticoagulants with greater use in this entity are low molecular weight heparins due to their anti-inflammatory properties, they reduce the formation of thrombin and with it the appearance of venous or pulmonary thromboembolic events. Studies derived from SARS-CoV 2 conclude that initial treatment with low molecular weight heparin (LMWH) reduces mortality by 48% at 7 days and 37% at 28 days, achieving a significant improvement in the blood pressure/oxygen fraction ratio. inspired oxygen (PaO2/FiO2) by mitigating the formation of microthrombi and associated pulmonary coagulopathy. Furthermore, in studies of critically ill patients, the use of LMWH decreased the inflammatory condition. For this reason, studies derived from COVID-19 use LMWH in some cases during admission in prophylactic doses (enoxaparin 40-60 mg/day) for at least 7 days [6]. In a controlled clinical trial study COVID-19 mild phase, 93% efficacy was reported when administering Rivaroxaban/Azithromycin/Ivermectin, so knowing the mortality would contribute to identifying the behavior in patients with COVID-19 mild phase [7]. Considering the objective of this study to estimate mortality in patients diagnosed with COVID-19 after a comparative treatment of early intervention in beneficiaries of the U.M.F 13 and U.M.F 20 of the I.M.S.S., during the period of March 2021 and February 2022.
Study Design
A non-experimental, cross-sectional-retrospectivecomparative- secondary data analysis study was carried out prior to the inconvenience of data management by the investigators of a single-blind randomized clinical trial conducted and registered in clinicaltrials [8].
Scope and Period of Study
This study was carried out in the Family Medicine Unit No. 20 and Family Medicine Unit No. 13 of the Mexican Institute of Social Security in the period between March 2021 and February 2022 in 114 patients, with data obtained from the database before referred.
Participants
Of the 114 patients, the inclusion criteria were considered: over 18 years of age with a positive confirmatory PCR test, male and female sex, beneficiaries of the Family Medicine Unit No.20 and Family Medicine Unit No.13, living status and dead. Exclusion criteria: carriers of severe COVID 19, pathological personal history of hematological diseases, allergies to macrolides and ivermectin or previous anticoagulant treatment, a hypothesis test with no difference in mortality percentage ≥ 3% in patients with a diagnosis of low COVID-19 a comparative early intervention treatment.
Variables
The measurements were made considering as an independent variable: age, sex, comorbidities (overweight, obesity, diabetes and arterial hypertension), marital status, schooling, number of days of COVID-19 illness and the dependent mortality between both groups of patients who received treatment formed by group A of 67 patients who received treatment with Azithromycin/Ivermectin/ Rivaroxaban and group B of 47 patients who received treatment with Azithromycin/Rivaroxaban, both groups had follow-up records made by video call daily for 14 days.
Ethical and Legal Aspects
Data were included in a registry approved by the ethics and health research committee.
Statistical Methods
Data were analyzed from a database in the statistical package Statistical Package for Social Sciences (SPSS) version 21, evaluating by comparative group of living and dead using frequency tables and percentages in variables such as age, sex, comorbidities (overweight, obesity , diabetes and arterial hypertension), marital status, schooling, applying the Pearson Chi-square test to these variables; In the number of days of COVID-19 illness with followup, survival analysis was used with the Wilcoxon (Gehan) test, considering statistically significant a value p < 0.05.
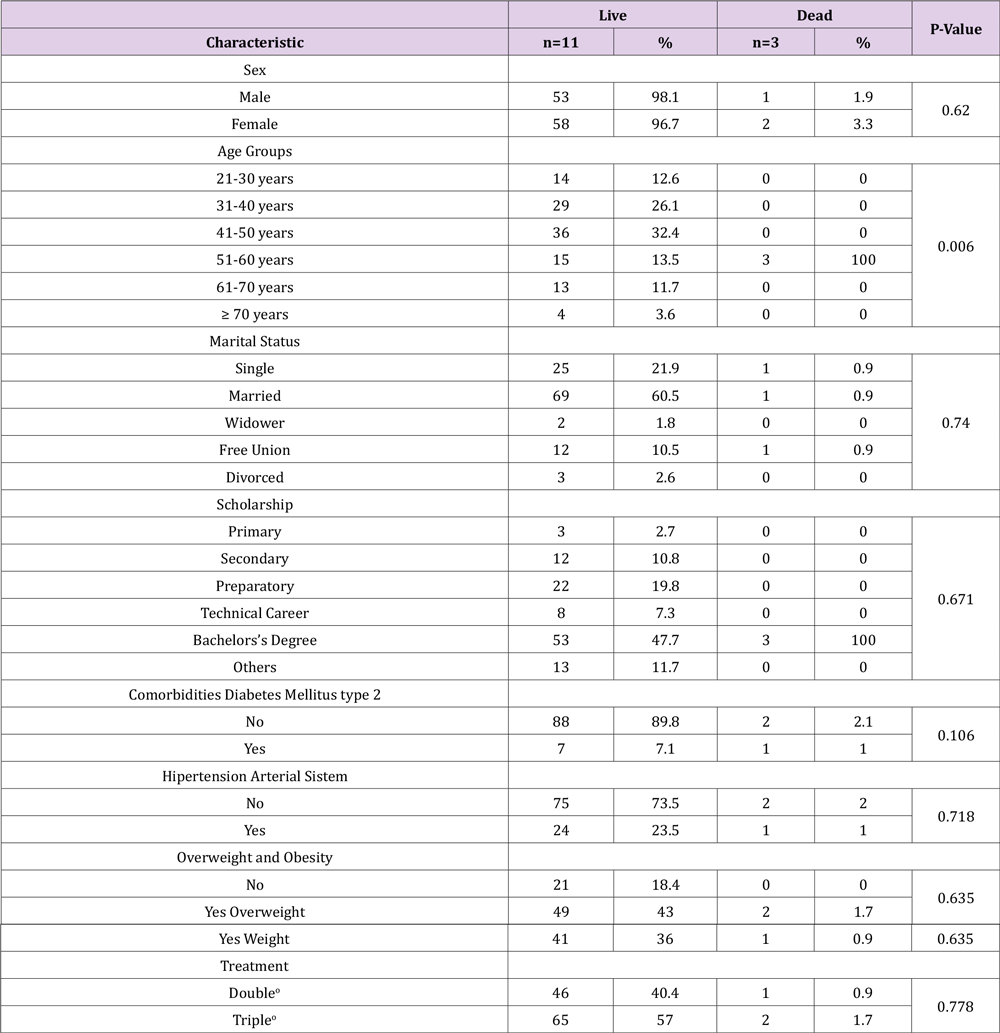
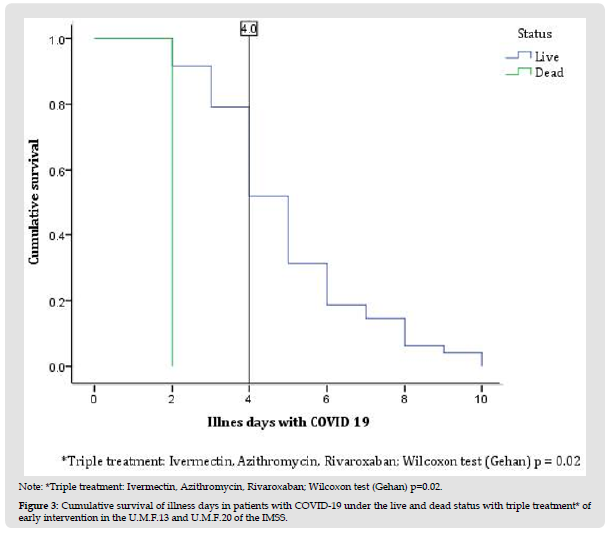
In this secondary data analysis study, 114 patients with COVID-19 divided into living (n=111) and dead (n=3) were analyzed (Figure 1); The female sex presented a higher percentage of mortality compared to the male sex, the age group 51 to 60 years presented 100% (n=3), compared to the other age groups, with a value p=0.006. Patients with no comorbidities such as diabetes and arterial hypertension had a higher mortality rate compared to those who did have comorbidity, with 2.1% and 2%, respectively; In relation to overweight, the percentage was higher compared to obesity and its absence, with 1.7%. A higher percentage of mortality occurred in patients who had triple treatment of COVID-19 patients with 1.7%, compared to dual therapy with 0.9% (Table 1). The time it takes for 50% of patients with COVID-19 to die during followup was 1.5 days (100%) compared to patients who survived with an interval of 4 to 5.2 days (31-57%), presenting at the age group of 51-60 years, overweight, patients without diabetes, without systemic arterial hypertension and triple treatment, with a statistically significant difference with value p= 0.011, p= 0.027, p= 0.018, p=0.019 and p= 0.020, respectively (Table 2). The median cumulative survival of days of illness in patients with COVID-19 under the living and dead status was 4 days, with a statistically significant difference p=0.005; presenting the same value in triple drug treatment with a p value = 0.02 (Figures 1, 2 & 3).
Table 1: Sociodemographic characteristics of live and dead patienrs daignosed with COVID-19 after a compartive treatment of early intervention in beneficiaries of the U.M.F 13 and U.M.F 20 of the I.M.S.S.
Note:
*Statistically significant Pearson Chi-square test p<0.05.
oDouble: Azithromycin, Rivaroxaban; Triple: Ivermectin, Azithromycin, Rivaroxaban.
Table 2: Mortality table with record of characteristics of the living and dead status, cumulative proportion and median of patients with COVID-19 in days of follow-up during the year 2021 at the U.M.F 13 and U.M.F 13 of the I.M.S.S.
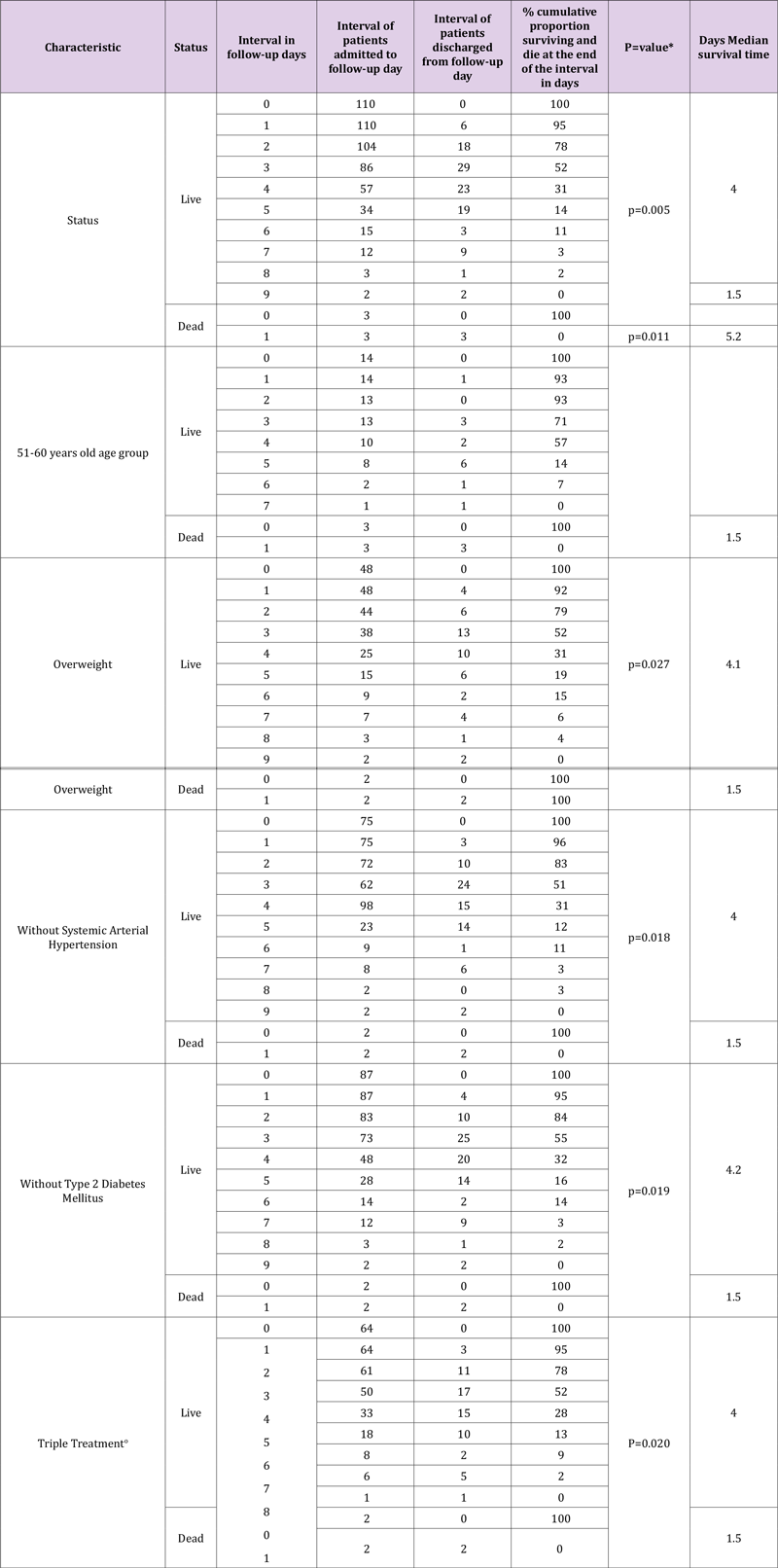
Note: *Wilicoxon (Gehan) test statistically significant p<0.05, oTriple: Ivermectin, Azithromycin, Rivaroxaban.
Figure 1 Note: Follow-up end day for the characteristics analyzed; oDMT2: Diabetes Mellites type 2; SAH: Systemic Arterial Hypertension; Triple: Ivermecdin, Azithromycin, Rivaroxaban. Figure 1: Flow of Participants to the study.
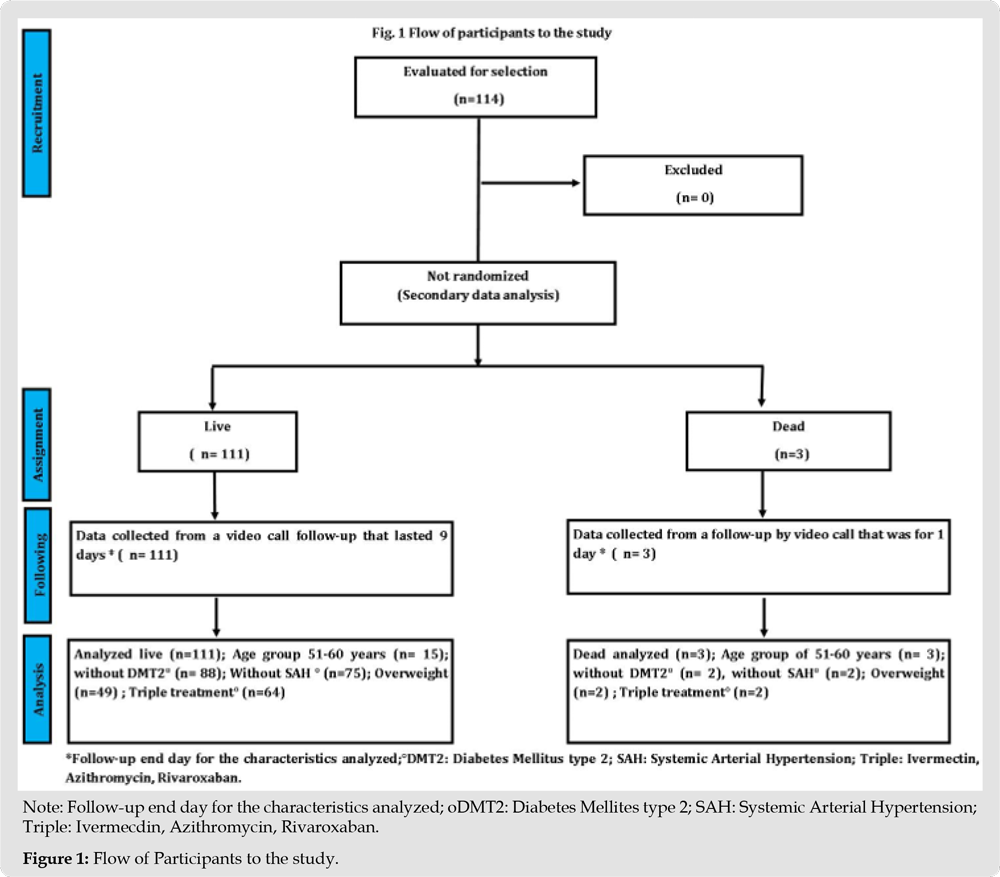
Figure 2 Note: *Wilcoxon test (Gehan) p=0.005. Figure 2: Cumulative survival of illness days in patients with COVID-19 under the status* live and dead in IMSS UMF and UMF 20.
Figure 3 Note: *Triple treatment: Ivermectin, Azithromycin, Rivaroxaban; Wilcoxon test (Gehan) p=0.02. Figure 3: Cumulative survival of illness days in patients with COVID-19 under the live and dead status with triple treatment* of early intervention in the U.M.F.13 and U.M.F.20 of the IMSS.
In the present study we identified the age group with the highest mortality between 51-60 years of age with 2.6% mortality, with a presence similar to that reported by (Carrillo Vega, et al. [9]) and an increase of up to 25% higher in the percentage proposed by (Palacio, et al. [10]) where the most affected age group was 45 to 64 years of age with a mortality record of 71.5%, followed by the age group of 65 years or older with 42.5%. There is a finding in relation to the percentage of mortality in the male sex found in our study contradicting the data provided by national statistics, to mention in a study where the early estimation of risk factors and mortality from COVID-19 according to the sex that men had a higher risk of death than women with OR= 1.53, p<0.001 [10]. In the result of mortality due to comorbidity, diabetes mellitus, systemic arterial hypertension and obesity each reached 1%, contrary to what was reported by some studies, as reported by (Ruiz Quiñonez, et al. [11]). Where it was found that one of the comorbidities associated with mortality is the presence of overweight with 66.7%, unlike the presence of obesity, arterial hypertension and diabetes mellitus with 33.3%, with this it is established that the high mortality rate in infected patients by SARS-Cov-2 is directly affected by the type of population, since in Mexico a significant proportion suffers from 2 or more comorbidities simultaneously; (Saenz José, et al. [12]) refers that the presence of underlying diseases increases the significant risk of mortality [11,12]. According to the database analyzed in the present study, dual and triple therapy for patients with COVID-19 showed a mortality of 0.9% and 1.7%, respectively. Being ivermectin part of the triple treatment as mentioned by (Cepelowicz Rajter, et al. [13]) in a review of 19 studies on the use of ivermectin in patients with COVID 19, reporting that the administration of ivermectin has been significantly associated with lower mortality in patients with SARS-CoV 2 disease and particularly in patients with alterations moderate to severe pulmonary [13]. (Khan, et al. [14]) reports a decrease in therapeutic failure with the administration of ivermectin in low doses [14]. Azithromycin was part of the two treatments (dual and triple) with a low prevalence of mortality; Rosenberg et. to the. refers to a 44% reduction in patients discharged with COVID-19 [15].
Rivaroxaban was also in both treatments and its relationship with the low mortality reported in this study; although there is low mortality in other conditions such as pulmonary embolism, [16] A study shows rivaroxaban with a positive interaction effect with antiviral drugs when indicated to patients with COVID-19, in addition to a study that considers the remote follow-up of patients with COVID-19 giving the same dose of rivaroxaban from the data of patients obtained from the present study [17,18]. (Gilberto CA, et al. [7]) refers to achieving in a survival table a median difference with dual and triple therapy greater than 25% in symptoms of patients with COVID-19 [7]. Finding a difference, according to graphs 1 and 2 of the present study, a median of 4 days in the follow-up of patients with COVID-19 in a living and dead state, the same value reported in the triple therapy given to these patients.
Within the limitations in the present study, the sample size (n=114) of the analyzed data was insufficient to obtain a statistically significant difference on the type of dual or triple therapy in relation to the living and dead state, so it could be one of the reasons why, according to the study hypothesis, a mortality difference of ≥ 3% was not achieved in patients diagnosed with COVID-19 between both treatments. The data in relation to comorbidities show a significant difference in patients with the absence of diabetes mellitus and arterial hypertension and overweight, this was due to the fact that the patient inclusion criteria in the original study did not contemplate homogeneity in these variables, so the diabetes mellitus, hypertension and obesity had a lower percentage of 7.1%, 23.5 and 36.0% respectively, compared to those without comorbidity.
In the present study, it was possible to achieve the objective of estimating a percentage of mortality in patients diagnosed with COVID-19 after a comparative treatment of early intervention, however, the difference in prevalence was lower than that proposed by the null hypothesis, which allows indicate that the mortality prevalence result obtained is excellent to consider further research in the future to define or not a therapeutic approach for patients with SARS-Cov2 to improve health.
To all the researchers of the original article for obtaining the data source that allowed the successful completion of this research study. All the authors of this study approve the publication of this paper.
The researchers of this article declare that there is no economic interest or conflict of interest.


