Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Marisela García Mendoza1, Martha Patricia Sierra Vargas2, Yazmin Debray Garcia2, Manolo Sibael Ortega Romero3, Jonathan Uriel Quevedo Martínez4, Victor Manuel Bautista de Lucio4 and Octavio Gamaliel Aztatzi Aguilar3*
Received: October 19, 2022; Published: November 08, 2022
*Corresponding author: Octavio Gamaliel Aztatzi Aguilar, Depto. de Toxicología. Centro de Investigación y de Estudios Avanzados del IPN. Av. Instituto Politécnico Nacional 2508, San Pedro Zacatenco, Gustavo A. Madero. 07360. CDMX, México
DOI: 10.26717/BJSTR.2022.47.007445
Aging is a natural process that causes irreversible histological changes leading loss of cell functions. Since the skin is exposed to the environment, it is thought that the most affected organ by aging is the skin, still morphological and functional changes occur in all of the internal organs. In this study ageing-related renal and testicular changes were aimed to investigate. Fourteen male Sprague-Dawley rats were divided into two groups: Group I was designated as sham-pinealectomized group and Group II was designed as pinealectomized (Px) group. Pinealectomized animals were considered as aged animals 6 months after the procedure. Kidney and testis samples were stained with Hematoxylin-eosin, Masson’s trichrome, Periodic Acid Schiff techniques, besides TUNEL and PCNA immunohistochemistry were applied. The most striking aging-related histological changes at testes were disorganization of the seminiferous epithelium, intra-tubular and inter-tubular edema, and basement membrane disruptions. Mean diameter of seminiferous tubules of aged rats was lower than that of young rats (p<0.05). Mean number of PCNA positive cells of the seminiferous tubules of aged rats was lower than that of young rats (p<0.05), conversely, mean number of apoptotic cells of the seminiferous tubules of aged rats was higher than that of young rats (p<0.001).
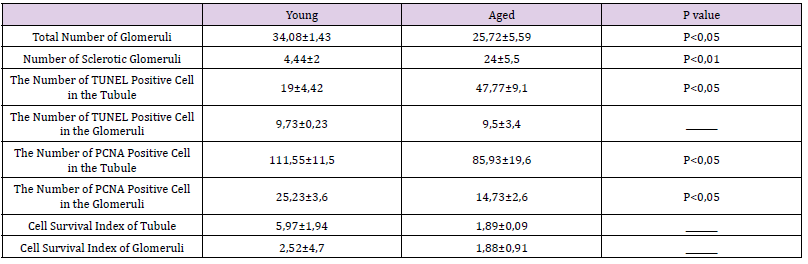
The most striking features of aged kidneys were tubular atrophy, tubular collapse, and glomerular sclerosis. Tubular and glomerular basement membranes seemed to be thickened. Brush border loss was also obvious. Mean number of healthy glomeruli of aged kidneys was lower than that of the young kidneys (p<0.05), conversely mean number of sclerotic glomeruli of aged kidneys was higher than that of the young kidneys (p< 0.001). Mean number of PCNA positive cells of the tubules and glomeruli of aged rats was lower than that of young rats (p<0.05), conversely, mean number of apoptotic cells of the tubules was higher than that of young rats (p<0.05).
Aging is a natural process that progresses over time, including irreversible changes triggered by various endogenous and environmental factors and leading to loss of cell functions (Hussein, et al. (1)). Age-related organ damage is characterized by morphological and functional changes caused by the accumulation of senescent cells. Since the skin is exposed to the environment, it is thought that the most affected organ by aging is the skin, still morphological and functional changes occur in all of the internal organs (Esrefoglu, et al. (2)). Testes and kidneys are two of the organs undergoing morphological and functional changes in the course of aging. Aging causes changes in hormone production, testicular morphology, spermatogenesis and spermiogenesis having negative impact on spermatozoon morphology and number, thus quality. Hypothalamic-pituitary-gonadal axis is affected, male reproductive functions are impaired (Gunes, et al. (3)). Aging-related atrophy has been reported in both humans and rodents leading impairment in spermatozoon number, motility, viability, semen volume and quality as well as deterioration of spermatozoon morphology (Alshinnawy, et al. (4,5)). Kidneys are one of the most affected organs in the course of aging. Aging is associated with significant changes in kidney structure and function as well. The renal cortical volume decreases, incidence of simple kidney cysts increases. Glomerular and tubular damage are common. At microscopic level, arteriosclerosis, glomerulosclerosis, interstitial fibrosis, and tubular atrophy which are histopathological clues for nephrosclerosis have been reported (Hommos, et al. (6)). Glomerular damage is represented by sclerotic changes including matrix expansion, narrowing or collapse of Bowman’s space, capillary collapse, and thickenning of glomerular and tubular basal membranes. Functionally, changes in the permeability of the glomerular capillary wall, glomerular filtration rate, tubular reabsorption and secretion capacities, urine concentration, production of kidney-dervied hormones have been reported (Esrefoglu, et al. (1)).
In healthy people, proliferation and apoptosis processes proceeds under the control of balanced mechanisms. Aging is accompanied with diminished proliferation and increased apoptosis. Imbalances in the proliferation and apoptosis processes are involved in numerous epithelial alterations. Normal spermatogenesis is regulated by spermatogonia proliferation and germ cell apoptosis. Aging-related testicular changes seem to be related with proliferation and apoptosis (Pastor, et al. (7)). Agedependent decline in renal repair in rodents has been attributed to the loss of proliferative capacity thus reduced epithelial proliferative reserve (Schmitt, et al. (8)). Conversely, apoptosis rate associated with up-regulation of several apoptosis-related markers has been found increased in aged rodent kidneys (Lee, et al. (9)).
At the present study we tried to investigate age-related histopathological changes and cellular proliferation and apoptosis status on kidney and testes tissues of Sprague-Dawley rats.
Experimental Protocol
Fourteen male 4-weeks-old Sprague-Dawley rats weighing 150–200g were placed in a constant temperature (21±2°C) and humidity (60±5%) controlled room in which a 12:12h light dark cycle was maintained. Animals were placed in cages two by two. The animals were divided into two groups: Group I (n=7) and group II (n=7) were designated as sham-pinealectomized and pinealectomized (Px) rat groups; respectively. Pinealectomy was performed to half of the rats under general anesthesia of ketamine/ ksilazin (30/15 mg) according to the method of Kuszak and Rodin (Kuszak (10)). Pinealectomy was confirmed by the histological evaluation of the gland. Pinealectomized animals were considered as aged animals 6 months after the procedure.
Histopathological Analysis
Kidney and testis samples were fixed in 10% buffered neutral formalin and prepared for routine paraffin embedding. 5 μm sections stained with hematoxylin-eosin (HE), Masson’s trichrome, and Periodic Acid Schiff (PAS) staining techniques were examined by a blind observer using a Nikon Eclipse i5 light microscope with a Nikon DS-Fi1c camera and the Nikon NIS Elements version 4.0 image analysis systems (Nikon Instruments Inc., Tokyo, Japan). Diameters of 10 seminiferous tubules per testis section was measured under 20X magnification. Glomeruli and sclerotic glomeruli numbers were counted on 5 area per section under 4X magnification.
Immunohistochemistry
Cell proliferation was detected by using cell proliferation detection kit (ThermoFisher Scientific, 991143). The mitotic cells were stained by using anti-PCNA (proliferating cell nuclear antigens) antibody. In order to evaluate apoptotic cells, terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) staining was performed by using in situ cell death detection kit (Merck Millipore, S7100). Thymus samples were used as positive controls. The number of PCNA positive and TUNEL positive cells were calculated in 10 seminiferous tubules under 40X magnification. The number of anti-PCNA positive and TUNEL positive cells were calculated in 10 renal tubules and 10 glomeruli under 40X magnification. A dynamic cell survival index (CSI) (proliferation/apoptosis rate) was calculated per section for all of the groups.
Statistical Analysis
Statistical analysis was done by using GraphPad Prism 8 program. Student’s T-test was performed in order to compare the results of two different groups. P<0.05 was considered as significant.
Testes
Testes of the young rats were as healthy as expected (Figures 1A & 1B). The most striking changes at aged testes were disorganization of the seminiferous epithelium, intra-tubular and inter-tubular edema, and basement membrane disruptions (Figures 1C & 1D). Additionally, vascularization seemed to be increased. Mean diameter of seminiferous tubules of aged rats (156.56±6.03) was lower than that of young rats (185.64±14.85) (p<0.05). Mean number of PCNA positive cells of the seminiferous tubules of aged rats (56,36±8,43) was lower than that of young rats (81,64±15,05) (p<0.05). (Figure 2 & Table 1). Conversely, mean number of apoptotic cells of the seminiferous tubules of aged rats (97,8±14,3) was higher than that of young rats (29,03±9,2) (p<0.001) (Figure 3 & Table 1). Cell survival index of young rats was higher than that of aged rats (Table 1).
Figure 1 A, B. Healthy testis histology is seen. Homogenously continued basal membrane is marked by arrows. C, D. Various changes are observed at aged testes. Disorganization of the seminiferous epithelium, intratubular edema (asterisks), intertubular edema (black asterisks), and basement membrane disruption (black arrows) are clear. E. Mean diameters of seminiferous tubules of young and aged rats are shown (p<0.05). H-E, PAS, Masson’s trichrome, PAS; respectively.
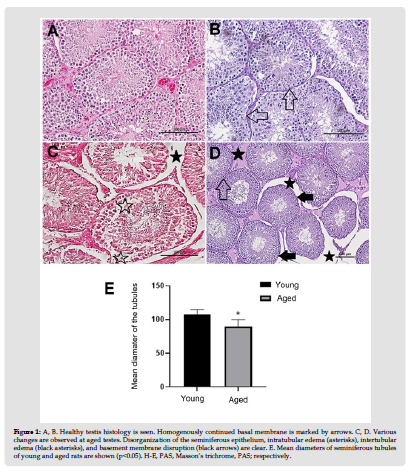
Table 1: Seminiferous Tubule Diameter, TUNEL and PCNA positive cell number and cell survival index in testicular tissue.
Kidneys
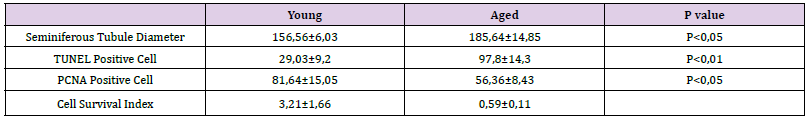
Kidneys of the young rats were as healthy as expected (Figures 4A & 4B). The most striking features of aged kidneys were tubular atrophy, tubular collapse, and glomerular sclerosis. Tubular and glomerular basement membranes seemed to be thickened. Brush border loss was also obvious (Figures 4C & 4D). Mean number of healthy glomeruli of aged kidneys (25.72±5.59) was lower than that of the young kidneys (34.08±1.43) (p<0.05). Conversely mean number of sclerotic glomeruli of aged kidneys (24±5.5) was higher than that of the young kidneys (4.44±2) (p< 0,001) (Figure 4E &Table 2). Mean number of PCNA positive cells of the tubules (85,93±19,6) and glomeruli (14,73±2,6) of aged rats was lower than that of young rats (111,55±11,5;25,23±3,6, respectively) (p<0.05) (Figure 5 & Table 2). Conversely, mean number of apoptotic cells of the tubules (47,77±9,1) was higher than that of young rats (19±4,42) (p<0.05). The mean number of apoptotic cells in the glomeruli of aged rats (9.5±3.4) was slightly lower than that of young rats (9,73±0,23), but not statistically significant. (Figure 6 & Table 2). Cell survival indexes regarding with the tubular epithelial cells and glomerular cells of young rats were higher than those of the aged rats (Table 2).
Figure 4 A, B. Healthy kidney histology is seen. Glomeruli (G) containing Bowman’s space and tubules containing visible lumens are normal in histological aspect. Thin glomerular basement membrane is marked by black arrow. Brush border is obvious. C, D. Various changes are observed at aged kidneys including glomerular (G) sclerosis, tubular atrophy (asterisk), collapse, basement membrane thickening (black arrow), and brush border loss. E. Mean numbers of healthy and sclerotic glomeruli at the sections from young and aged animals are shown (p<0.05, p<0.001; respectively). H-E, PAS, H-E, PAS; respectively.
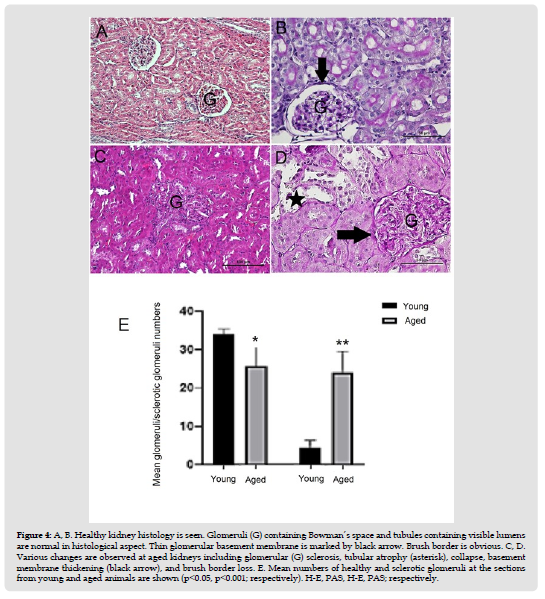
Figure 5 Distribution and mean number of PCNA + cells of tubules and glomeruli (respectively) are observed. Microscopic figures reveal loss of PCNA + cells at the renal tubules and glomeruli of aged kidneys (p<0.05).
Figure 6 Distribution and mean number of TUNEL + cells of tubules and glomeruli (respectively) are observed. Microscopic figures reveal increase in TUNEL + cells especially at the epithelium of the distal tubules (asterisks) of aged kidneys (p<0.05).
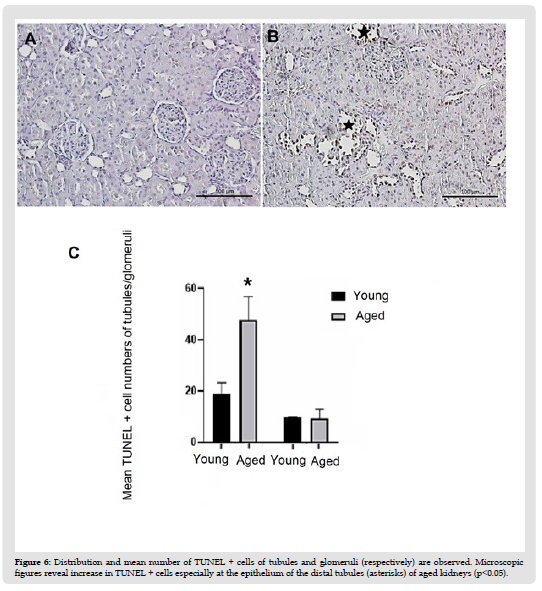
Table 2: Total number of glomeruli, number of scleratic glomeruli, TUNEL and PCNA indeks in tubules and glomeruli and Cell Survival index of tubules and glomeruli in kidney tissue.
Aging is a natural, progressive, and inevitable biological process characterized by gradual decline of cellular function and progressive structural changes in various organ systems (Denic, et al. (5)). Some theories associate several factors, such as changes in metabolic control and gene expression patterns, and production of high levels of reactive oxygen species (ROS), to aging and ageing rate. Aging is associated with molecular, structural, and functional changes in various organs, including testes and kidneys (Hommos, Hussein et al (6, 4)). Both male and female reproductive capacities decrease with age although striking in females. Since spermatogenesis and spermiogenesis proceed until very old ages, it is assumed that men can have children throughout their lives. Nevertheless, advancing paternal age leads to faulty sperm production leading to impairment of reproductivity (Gunes, et al. (3)). Histomorpholoical studies showed a clear decrease in the number of germ cells and Sertoli cells with aging. Thickness of tunica propria of the seminiferous tubules increases, thickness of the seminiferous epithelium decreases, thus tubule diameter decreases during aging. Increase in neovascularization leads to testicular fibrosis. Fibrosis disrupts nitration of the epithelium leading to tubule atrophy (Esrefoglu, Gunes et al; Johnson) (2,3,11)). At the present study we detected disorganization of the seminiferous epithelium, intra-tubular and inter-tubular edema, and basement membrane disruptions. Additionally, vascularization seemed to be increased. Mean diameter of seminiferous tubules of aged rats was lower than that of young rats (p<0.05). Mean number of PCNA positive cells of the seminiferous tubules of aged rats was lower than that of aged rats (p<0.05). Conversely, mean number of apoptotic cells of the seminiferous tubules of aged rats was higher than that of young rats (p<0.001). Cell survival index of young rats was higher than that of the older rats suggesting higher mitosis rate but lower apoptosis rate. Aging is accepted to be accompanied by diminished proliferation and increased apoptosis; the latter occurring in specific states of the seminiferous cycle and considered the cause of epithelium involution (Pastor, et al. (12)). However, some studies in rat seemed to show that stem spermatogonia proliferative activity is apparently normal in aged testis, suggesting that the possible cause of germ cell loss during aging is the apoptosis process (Schoenfeld, et al. (13)). In our study proliferation rate of tubule epithelium of aged rats was lower than that of the young rats (p<0.05). In a Ki-67 expression study in man, a decrease in the proliferating spermatogonia percentage has been documented in aged individuals with respect to young ones. Interestingly, in aged men a significant diminution in the apoptotic spermatogonia percentage accompanied by an increase in apoptotic primary spermatocytes compared with young individuals has been observed (Kimura, et al. (14)).
In our study, regarding apoptosis rate of the whole seminiferous tubule epithelium, apoptosis was found increased versus young rats (p<0.001). The results available to date indicate that both decreased proliferation and germinal apoptosis are responsible for germ cell loss and tubular atrophy in aging testes (Barnes, et al. (9, 12,6,23)). Relative apoptosis activity of spermatogonia and spermatocytes increases in ageing. There is disagreement concerning whether the decrease in proliferation is only due to changes of the microenvironment of the spermatogonia stem cells or whether aging also directly affects the proliferative activity of spermatogonia stem cells (Pastor, et al. (12)). Recently, some studies have shown that aging not only affects the somatic cell environment, but also the spermatogonial stem cell activity (Zhang, et al. (16)), while others suggest that infertility in old mice results from deterioration of the spermatogonial stem cell niche and failure to support an appropriate balance between stem cell self-renewal and differentiation (Ryu, et al. (17)).
Like the other organ systems, urinary system also suffers from aging characterized by anatomical, microscopic, and functional changes (Esrefoglu (18)). Ageing is associated with glomerular and tubular damage. Glomerular damage is represented by sclerotic changes such as matrix expansion, narrowing or disappearance of the Bowman space, capillary collapse, and thickening of glomerular basement membrane. Esrefoglu, et al.(19,2)). Tubular damage is represented by tubular dilatation and tubular atrophy, epithelial degeneration, vacuolization, and tubular cast formation including thyroidization. Interstitial cell infiltration, interstitial fibrosis and congestion are also common (Esrefoglu, et al. (19,2)). At electron microscopic level microvilli loss and disorganization are clearly observed (Esrefoglu (18,19)). In the present study we detected tubular atrophy, tubular collapse, glomerular sclerosis, tubular and glomerular basement membrane thickening, and brush border loss. Brush border loss implies microvilli loss since brush border is an indicator of tightly packed microvilli at the apical surface of the epithelium of proximal tubules. Mean number of healthy glomeruli of aged kidneys was lower than that of the young kidneys (p<0.05). Conversely mean number of sclerotic glomeruli of aged kidneys was higher than that of the young kidneys (p< 0,001). Mean number of PCNA positive cells of the tubules and glomeruli of aged rats was lower than that of young rats (p<0.05). Conversely, mean number of apoptotic cells of the tubules and glomeruli of aged rats was higher than that of young rats (p<0.05). Cell survival indexes regarding with the tubular epithelial cells and glomerular cells of young rats were higher than those of the aged rats.
Nephrosclerosis and glomerular basement membrane thickening have been reported in previous studies (Nyengaard, Bendtsen (20)). Nephrosclerosis is characterized by global glomerulosclerosis, tubular atrophy, and interstitial fibrosis (Sethi, et al. (21)). Aging-related glomerulosclerosis identified in autopsy studies is present in more than 70% of people older than 40 years (Kaplan, et al. (22)). After age of 30, approximately 6.000-6500 nephrons per year is lost due to nephrosclerosis or more specifically glomerulosclerosis (Denic, et al. (11,24)). A recent study of 1638 healthy kidney donors showed that the number of nephrons decreased by 48% in 70-75-year-old persons compared to 18-29-year-old persons, while the sclerotic glomeruli increased by 15% globally (Denic, et al. (24)). In our study we clearly detected significant loss of healthy glomeruli and increase in sclerotic glomeruli in aged rats. A possible explanation for the progressive increase in sclerotic glomeruli with age is glomerular ischemia secondary to the changes in renal blood flow that occur with age (Dontas, et al. (25)). Nephrosclerosis has been found to be associated with arteriosclerosis (Silva (26-28)).
A small fraction of the renal cell has the ability for proliferation and repair (Cardani, Zavanella (29)). It has been shown that aged rodent kidneys display a reduced epithelial proliferative reserve contributing to age-dependent decline in renal repair (Cardani, Zavanella (29,8)). (Cardani, et al. (29)) reported a progressive increase in proliferation rates in rats from 4 and 6-10 months of age. In 23-month-old rats, proliferative activity appeared to be reduced.
The levels of growth factor including epidermal growth factor, insulin-like growth factor, vascular endothelial growth factors decline with age (Famulski, Halloran (30)). These factors stimulate cellular proliferation. (Lee, et al. (31)) showed that aging is associated with an increased expression of Bax, a decrease of Bcl- 2, cytochrome c release, and the activation of caspase-3, which are essential components of the apoptosis cascade process. Conversely (Cardani, et al. (29)) found no age-related variations in apoptotic indices. We detected significant decrease in proliferation rates and significant increase in apoptosis rate. Cell survival index of aged rats was also significantly lower than that of young rats. Apoptosis and proliferation are the principal podocyte changes leading sclerosis and loss of nephrons (Camici, et al. (32)). Although we did not detect a significant change in terms of glomerular apoptosis, we detected a significant decrease of glomerular proliferation rate (p<0.05). Resulting podocyte loss should be related with focal denudation of the glomerular basement membrane that is the origin of focal adhesions of the glomerular tuft to the outer leaflet of Bowman’s capsule (Petermann, et al. (33)).
As a conclusion, although, mainly the skin is thought to be effected by aging, aging leads to striking microscopical changes that also lead to life-threatining and reproduction-threatining changes in kidney and testis; respectively.
This study was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) (Project no: 213S106).


