Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Marisela García Mendoza1, Martha Patricia Sierra Vargas2, Yazmin Debray Garcia2, Manolo Sibael Ortega Romero3, Jonathan Uriel Quevedo Martínez4, Victor Manuel Bautista de Lucio4 and Octavio Gamaliel Aztatzi Aguilar3*
Received: October 19, 2022; Published: November 08, 2022
*Corresponding author: Octavio Gamaliel Aztatzi Aguilar, Depto. de Toxicología. Centro de Investigación y de Estudios Avanzados del IPN. Av. Instituto Politécnico Nacional 2508, San Pedro Zacatenco, Gustavo A. Madero. 07360. CDMX, México
DOI: 10.26717/BJSTR.2022.47.007444
Diabetic Retinopathy (DR) is a consequence of a lack of glucose control and glucotoxicity. Oxidative stress (OxS) is related to tissular dysfunction. The present study evaluated the enzymatic activity of myeloperoxidase (MPO), Arginase, Glutathione S-Transferase (GST), and the metabolite Methylglyoxal (MGO) in the ocular surface lavage (OSL) and serum of patients at different DR stages. We determined the relationship of biomarkers with DR stage, and with local and systemic responses. Clinical data included age, diabetes diagnostic age, intraocular pressure (IOP), and systemic glucose. Biomarkers were analyzed by colorimetric assays. MPO, MGO, and arginase in OSL showed a statistical difference among DR stage groups. Serum MPO, GST, and arginase were significantly lower in DR patients. Biomarker levels in OSL showed a positive correlation with clinical data. Serum biomarkers showed an inverse correlation with respect to the OSL evaluation. A statistically significant increase in IOP was observed in the 4th quartile of serum MGO level. Multivariate analysis shows that the MGO levels in OSL depend on serum GST levels. The present study observed the contribution of biochemical changes on methylglyoxal and OxS biomarkers in OSL concerning systemic evaluation in DR.
Keywords: Oxidative Stress; Diabetic Retinopathy; Ocular Surface Lavage; Glucotoxicity; Methylglyoxal
KAbbreviations: Diabetic patients without Diabetic Retinopathy (wDR); Diabetes Mellitus (DM); Diabetic Retinopathy (DR); Non-Proliferative Diabetic Retinopathy (NPDR); Proliferative DR (P-DR); Oxidative Stress (OxS); Methylglyoxal (MGO); Glutathione S-Transferase; Myeloperoxidase (MPO); Advanced Glycation end Products (AGEs); Ocular Surface Lavage (OSL)
Diabetes mellitus (DM) is a prevalent disease worldwide and is attributed to many factors that include sedentarism, malnutrition, genetics, and environmental exposure. DM patients are characterized by several complications, which progress with time after the first diagnosis due to high concentrations of glucose that generate glucotoxicity. These complications can be treated by reducing glucose serum levels through nutritional vigilance and pharmacological treatments. A common condition in DM patients is macrovascular and microvascular affections, which contribute to a high risk of cardiovascular disease and loss of some senses such as skin sensitivity, hearing, and vision due to microvascular injuries. Diabetic Retinopathy (DR) is the most frequent cause of blindness in DM patients. It progresses from the age of diagnosis and with poor glycemic control. The worldwide prevalence in 2011 was estimated to be 126.6 million cases, and it is estimated to increase to 191.0 million by 2030 [1]. Prado serrano, et al. [2] reported the prevalence in Mexico from 13,670 cases examined, where 29% (3,965 cases) did not have DR, 71% (9,705) presented DR, 37% (3,591) had non-proliferative DR (NP-DR), and 63% (6,114 cases) showed proliferative DR (P-DR) [2].
Oxidative stress (OxS) has been reported to play an important role in several ocular diseases including dry eye, corneal inflammation, cataract, age-related macular degeneration, and retinopathy, to mention a few. OxS occurs as an imbalance between the production of reactive radicals and antioxidant defense. OxS is implicated in the development and progression of acute and chronic diseases. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced during endogenous processes, e.g., mitochondrial respiration, inflammation, and P450-mediated metabolism. Radicals can contribute to protein carbonylation, lipoperoxidation, oxidative degradation of saccharides, and DNA adducts and breakdowns. OxS biomarkers have been evaluated in non-conventional biological matrices in the eyeball, such as tears, aqueous humor, and vitreous humor, to explain their relationship with ophthalmological diseases [3]. In addition, proteomic and metabolomic studies have been carried out to explain differential changes in eye diseases [4,5]. However, an epidemiological evaluation of biochemical OxS biomarkers at different stages and their relationship with systemic and local effects is necessary to address further treatments and molecular targets to diagnose, determine the prognosis, stop the progress of the diseases and monitor them. Poor has been the study of nonconventional- biochemical OxS biomarkers in the knowledge of the biochemical surface of eye, to mention Methylglyoxal (MGO), Arginase (EC 3.5.3.1), glutathione S-transferase (GST; EC 2.5.1.18) and Myeloperoxidase (MPO; EC 1.11.2.2), which are involved in the biochemical changes in the physiopathology of diseases such diabetes.
The MGO is a dicarbonyl compound that is a precursor of Advanced glycation end products (AGEs), is a potent reactive molecule able to induce non-enzymatic glycation in lysin, cysteine, and arginine, forming AGEs. MGO is an indicator of glucotoxicity. This has been indirectly evaluated in tears and the human lens by the detection of MGO adducts with amino acids (e.g., cysteine and arginine) in patients with DR and cataract [6]. The presence of Arginase (EC 3.5.3.1) has been reported in vitreous humor and tears, but the biochemical function of this enzyme in the eye is poorly understood [7]. However, the accumulation of arginine, the substrate of arginase, has been observed in experimental corneal herpes. Low arginase content in rabbit tears supplemented by arginase applied as eyedrops has been observed to result in the cure of the herpetic process [8]. The GST enzyme family (EC 2.5.1.18) is involved in the detoxification of xenobiotic compounds and endogenous metabolites. An in-depth proteomic analysis was carried out in human tears, where the GST isoforms reported included GSTP1, GSTA2, GSTZ1, GSTO1, and GSTK1 [5]. Perioperative dry eye syndrome is a common ocular complication of long-term general anesthesia that can lead to permanent damage to the cornea and disturbance of visual function, up to a total loss of vision, where an increase in GST enzyme activity has been reported after 3 to 72 h of the postanesthetic period [9].
In addition, GST can conjugate intermediate metabolites of oxidized macromolecules, such as malondialdehyde, but GST is a target of glycation by MGO and the adduct is linked to GST inactivity [10]. MPO (EC 1.11.2.2) is a heme-containing peroxidase considered to be a specific marker for neutrophils. MPO catalyzes the formation of reactive oxygen intermediates, including hypochlorous acid (HOCl). The MPO/HOCl system plays an important role in microbial killing by neutrophils [11]. MPO enzymatic activity has been evaluated in serum and tears of patients affected by seasonal allergic conjunctivitis, vernal keratoconjunctivitis, atopic keratoconjunctivitis, giant papillary conjunctivitis, and bacterial conjunctivitis. MPO serum levels do not increase with any allergic condition and do not correlate with the severity of ocular signs and symptoms. MPO correlates with the clinical score of vernal keratoconjunctivitis, but tear MPO has been observed to be significantly higher in patients with bacterial conjunctivitis [12]. The objective of the present study was to evaluate the enzymatic activity of MGO, Arginase, GST, and MPO in the ocular surface lavage (OSL) and serum of DM patients at different the DR stages. We also determined the relationship of the biochemical OxS biomarkers with DR stage and the local and systemic response.
Subject Selection and Study Design
We conducted the study under the ethical principles of the Declaration of Helsinki. Patients were invited to a programmed clinical consult at the Instituto de Oftalmología “Fundación de Asistencia Privada Conde de Valenciana I.A.P.® The institutional Ethics Committee granted ethical approval, approval number CEI- 2016-08-03, and written informed consent was obtained from all the subjects involved in this study. Patients with DM2 were recruited for the present study. All patients were diagnosed according to the WHO diagnostic criteria for type 2 diabetes and were treated with oral hypoglycaemic agents and/or insulin. Patients with acute and chronic ophthalmological or general inflammatory disorders, glaucoma, age-related macular degeneration, high myopia, occlusion of a retinal artery or vein, ocular ischemic syndrome, eye surgical interventions in the last three months, panretinal photocoagulation or antiangiogenic application for DR, tumors, severe atherosclerosis with clinically proven myocardial infarction, strokes or other vascular incidents, and hepatic or renal insufficiency were excluded. Healthy, age-matched subjects were included in the control group. None of the control subjects were taking any medication.
Myeloperoxidase Activity Assay
Myeloperoxidase (MPO; EC 1.11.2.2) produces hypochlorous acid (HOCl) from hydrogen peroxide and chloride anion. The MPO assay was adapted to the microplate format from Suzuki, et al. [13]. Samples were placed in a 96-well microplate at a volume of 10 μl and incubated with 90 μl of a reaction mixture (0.168 mM 3,3 ‘, 5,5′-tetrametilbencidina, 0.0126 mM H2O2 in PBS 50 mM pH 7.4) at 37 °C for 10 min. [13]. The reaction was stopped with 150 μl Acetate buffer 0.4 M pH 3 and read at a single wavelength of 590 nm in a microplate reader. MPO enzymatic activity is referred to as 1 U for every 0.1 change in absorbance.
Arginase Activity Assay
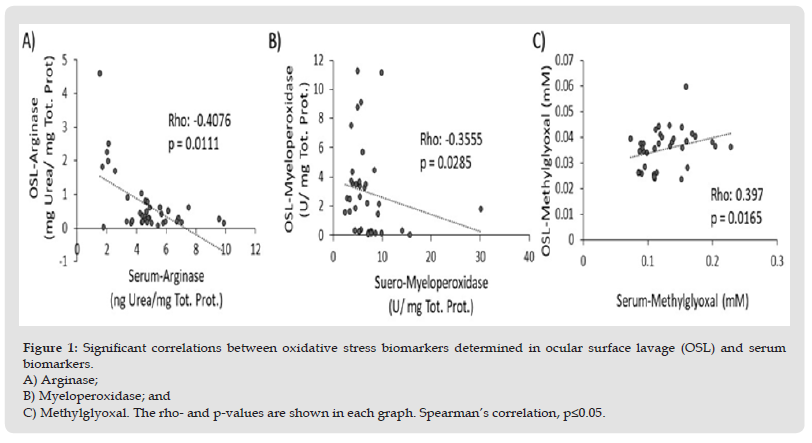
Arginase (EC 3.53.1) catalyzes the hydrolysis of L-arginine to L-ornithine and urea. The method was adapted to the microplate format from Corraliza, et al. [14]. Briefly, the samples were diluted to 1:10 directly with saline phosphate buffer (0.1 M pH 7.4: BioWhittaker Lonza Cat. No. 17517Q) and incubated at 55°C for 10 min. to activate the enzyme. The sample aliquots (50 μl) were then incubated for 1 hour at 37°C with L-Arginine to reach a final concentration of 0.25 M in a final volume of 100 μl. The final reaction was carried out by heating the samples at 100 °C for 45 min in darkness pre-mixed with a reaction mixture that was composed of 9% isonitrosopropiophenone dissolved in ethanol and an acid mixture (H2SO4, H3PO4, H2O; 1:3:7 v/v) in a proportion of 1:15. The microplates were kept at room temperature for 15 min. before performing the readings at 540 nm [14]. Urea was used as an internal control to construct the standard curve (Figure 1).
Figure 1 Significant correlations between oxidative stress biomarkers determined in ocular surface lavage (OSL) and serum biomarkers. A) Arginase; B) Myeloperoxidase; and C) Methylglyoxal. The rho- and p-values are shown in each graph. Spearman’s correlation, p≤0.05.
Methylglyoxal Determination
The colorimetric method was used to quantify methylglyoxal in OSL according to Kwok, et al. [15]; the 2, 4-dinitrophenylhydrazine (DNPH) Alpha-keto acid method was coupled to the microplate method. Briefly, the samples (20 μl) were mixed with DNPH reagent (0.9 mM in 1N HCl) and incubated at 37 °C for 10 min. Subsequently, NaOH 1.5 N was added, and the reaction was shaken and left for 5 min. to develop the coloration. The MGO solution (Cat. No M0252, Sigma Aldrich) was used as a standard to build a calibration curve, which was processed in the same way as the samples [15]. Absorbance was determined at 540 nm and concentrations were calculated by interpolating to a linear regression equation.
Glutathione S-Transferase Activity Assay
The enzymatic GST assay (EC 2.5.1.18) was performed according to the method by Habig, et al. [16] using chlorodinitrobenzene (CDNB) (Sigma Aldrich, Steinheim, Germany) as a substrate and reduced glutathione (Sigma Aldrich, St Louis, USA). The formation of GST–CDNB conjugate was monitored at 340 nm [16]. Total protein concentration was measured according to the method by Lowry, et al. [17] and absorbance was determined at 570 nm using bovine serum albumin (BSA, Sigma Aldrich) as a standard [17].
Statistical Analysis
Results are presented as the median and 25th-75th percentile range for continuous variables. A Kruskal Wallis test was used to compare the differences between ocular stages. Spearman’s correlation coefficient was used to express the relation between oxidative stress biomarkers and the clinical variables. A multivariate robust linear regression analysis was performed to describe the responses evaluated in the OSL. All statistical analyses were performed in STATA Version 12.0 statistical software package (StataCorp LP). A p-value < 0.05 was considered significant in all analyses (Figure 2).
Figure 2 Serum methylglyoxal concentration is related to intraocular pressure (IOP). A) A marginal correlation was observed between serum methylglyoxal and IOP, a Spearman’s correlation test was used B) Serum-Methylglyoxal was divided into quartiles to compare IOP, a Kruskal-Walli’s test followed by a Dunn’s post hoc test was used p<0.05.
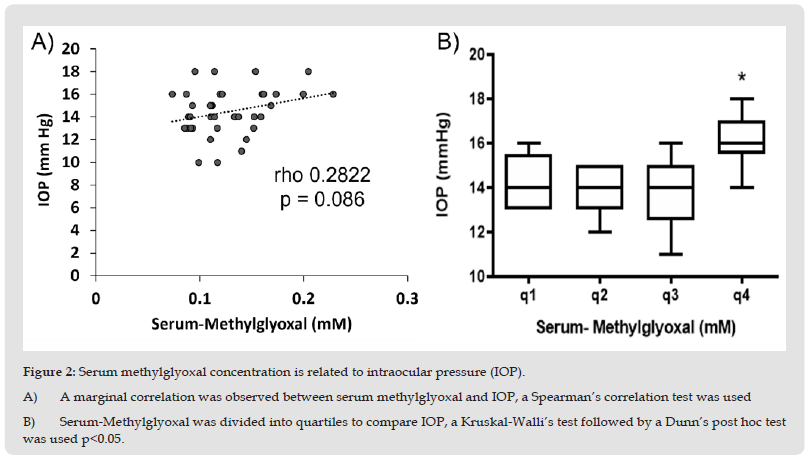
The general clinical characteristics of the patients showed significant statistical differences in age, DM- Time to diagnosis, and fasting systemic glucose. The evaluation of biochemical OxS biomarkers in OSL showed a significant statistical increase in MGO, Arginase, and MPO at all DR stages with respect to the control group. However, serum levels of Arginase, MPO, and GST showed a statistically significant decrement (Table 1). Biochemical OxS biomarkers, such as Arginase, MPO, and MGO, evaluated in the OSL had a positive and statistically significant correlation with fasting systemic glucose, but a negative and statistically significant correlation was observed between serum Arginase, MPO, and GST and fasting systemic glucose (Table 2). The biochemical OxS biomarkers MPO and MGO in OSL showed a positive and statistically significant correlation with DM-time to diagnosis. Nevertheless, the serum OxS biomarkers Arginase, MPO, and GST showed a negative and statistically significant correlation with DM-Time to diagnosis (Table 2). A statistically significant correlation between OSL and serum OxS biomarkers was observed. A negative correlation was observed between OSL and serum Arginase and MPO and a positive correlation was observed between OSL and serum MGO concentrations. In addition, a marginal correlation was observed between IOP and OSL MGO levels (p=0.08). To corroborate this relation, MGO levels were divided into four quartiles to compare the influence of MGO levels on IOP. A statistically significant difference was observed in the fourth quartile with high levels of IOP.
Table 1: Descriptive characteristics and oxidative stress biomarker levels in the ocular surface lavage and serum of type 2 diabetes mellitus patients at different retinopathy stages.
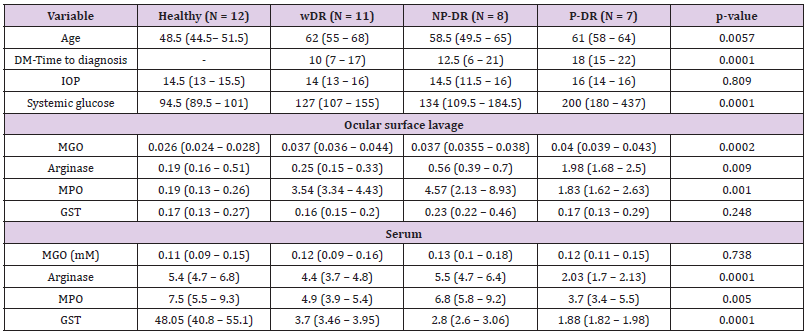
Note: Age is expressed in years; DM-Time to diagnosis is expressed in years; Intraocular pressure (IOP) is expressed in mmHg; Systemic glucose is expressed in mg/dL; Total protein is expressed in mg/ml; Methylglyoxal (MGO) is expressed in mM; Arginase is expressed in mg Urea/mg total protein; Myeloperoxidase (MPO) is expressed in U/mg total protein; Glutathione S-Transferase (GST) is expressed in pmol/min/mg total protein.
Table 2: Correlation of oxidative stress biomarkers in serum and ocular surface lavage with systemic glucose and diabetes mellitus time to diagnosis.
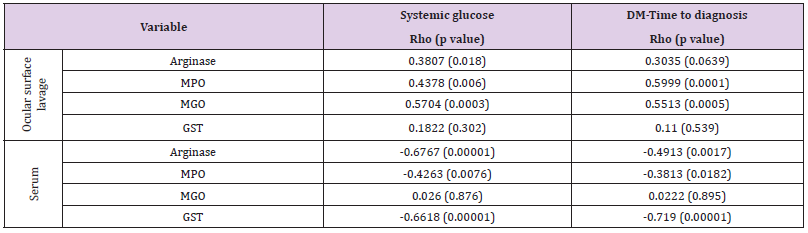
Note: Systemic glucose is expressed in mg/dL; DM-Time to diagnosis is expressed in years; Arginase is expressed in mg Urea/mg total protein; Myeloperoxidase (MPO) is expressed in U/mg total protein; Methylglyoxal (MGO) is expressed in mM; Glutathione S-Transferase (GST) is expressed in pmol/min/mg total protein.
A multivariate robust linear regression was used to determine what factors contribute to the increase in MGO in OSL. The results of the multivariate analysis showing the statistical differences and best-fit R2 values adjusted to the variables that contribute to the increase in MGO in the OSL can be observed in (Table 3). In Model 1, there is an increase of 0.0237 mM in MGO in the OSL for every increase in systemic glucose, and this effect is dependent on age, with an increase of 0.345 mM for every year. In Model 2, we observed that the increase in MGO in the OSL is dependent on serum GST, where there is a decrease of 0.202 mM in MGO in the OSL for every increase in serum GST, and the increase in MGO in the OSL is dependent on DM-Time to diagnosis, in both models, sex did not affect the increase in MGO.
Table 3: Multivariate robust linear regression for Methylglyoxal (MGO) in the ocular surface lavage (OSL).
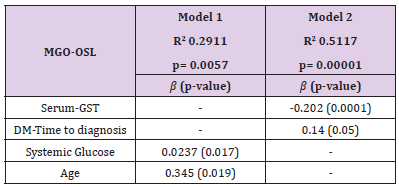
Note: Methylglyoxal is expressed in mM; Glutathione S-Transferase (GST) is expressed in pmol/min/mg total protein; Diabetes mellitus (DM) – Time to diagnosis is expressed in years; Systemic glucose is expressed in mg/dL; and age is expressed in years.
Diabetes mellitus is a worldwide epidemic problem with a highcost treatment and several health complications when blood glucose is not controlled. Blindness is a very frequent health complication in DM patients and DR is a consequence of microvascular damage in the retina that precedes blindness. OxS has been implicated in the progression of DR as a consequence of high uncontrolled blood glucose levels and subsequent glucotoxicity. Non-conventional samples, such as tears, aqueous humor, and vitreous humor, have been used to evaluate oxidative damage in the eye. The present study used ocular surface lavage as a matrix to evaluate OxS because DR patients suffer from dry eye. The evaluation of biochemical OxS biomarkers in OSL is a successful and available approach that can be used to evaluate the progression of eye damage. We observed that the patients with advanced DR, P-DR, showed a long DM-time to diagnosis and high fasting blood glucose. The OxS biomarkers evaluated in OSL showed the same pattern, except for the GST biomarker. Interestingly, OxS biomarkers in serum showed the inverse pattern, apart from MGO. In this sense, patients with advanced DR have a lower systemic activity of Arginase, MPO, and GST, which are biomarkers implicated in nitric oxide availability, innate defense mechanisms, and xenobiotic metabolism, respectively.
We observed that OxS biomarkers in the OSL correlate positively with fasting blood glucose and DM-time to diagnosis, but OxS biomarkers in serum show negative correlations. Based on this observation, OxS in peripheral compartments with high microvasculature, such the eye, is enhanced by the time course of the disease and glucose concentrations. However, it is important to highlight that the decrease in serum Arginase, MPO, and GST correlates with fasting blood glucose and DM-time to diagnosis, which implies a system compromised by chronic uncontrolled glucose. In addition, one objective of the present study was to compare the response to biochemical OxS biomarkers of local tissue (conjunctive) and systemic circulation (serum), and determine if there is a relation between the two involved in the progression of DR. A systemic decrease in Arginase and MPO promotes an increase in their enzymes in OSL, demonstrating the impact of central circulation effects on the eye. On the other hand, the positive correlation between serum and OSL MGO concentrations suggests that the oxidative degradation of saccharides in the eye is partly due to blood circulation.
Although we did not observe statistical differences in IOP between DR stages, serum MGO showed a tendency to correlate with IOP. However, the patients recruited in the present study did not have an IOP above the cut-off range of 21-22 mmHg to consider that they had ocular hypertension. Nevertheless, in the fourth quartile of MGO concentration, we observed patients with the highest IOP values (above 15 mmHg), which are above those reported in Latin American populations, where the mean IOP values are within the range of 14.4-15.6 mmHg [18]. Therefore, systemic MGO concentrations contribute to the increase in eye pressure, probably favoring changes in the production, circulation, and drainage of the ocular aqueous humor by affecting the permeability of microcirculation through endothelium damage. The multivariate analysis suggests that the increase in MGO in OSL with age and blood glucose is a natural process of aging under uncontrolled glucose. Nevertheless, MGO in OSL decreased with an increase in serum GST and with DM-time to diagnosis. Interestingly, GST activity decreased with the progression of DM and showed lower levels in the patients with P-DR. The detoxication function of GST plays a crucial role in the protection from xenobiotics, but glucotoxicity appears to be involved in GST regulation and the development of other diabetic complications such nephropathy [19]. Our results suggest that MGO, the main metabolite related to glucotoxicity, contributes to DR and its concentration in OSL is dependent on the central control of glucose and the enzymatic activity of GST. The present study has the limitation of the small number of participants which limited the statistical power of tests. Also, the small OSL sample limited the number of biochemical assays, in addition, we did not have other data available of nutritional state and blood chemistry that were not considered to give more strength in statistical relationships.
The present pilot study demonstrates the differential contribution of biochemical changes on OxS biomarkers in OSL concerning a systemic evaluation, and their correlation in the different stages of DR, which is mainly explained by glucotoxicity on serum GST activity concomitant with an increase in MGO concentrations in OSL.
The authors gratefully acknowledge the equipment and technical support of the Instituto Nacional de Enfermedades Respiratorias Ismael Cosío Villegas.
The authors report there are no competing interests to declare.
This work was supported by the CONACYT under grant FOSIS-273146.
The datasets generated during and/or analyzed during the current study are available in the ww.figshare.com repository.


