Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Luisetto Mauro*, Edbey K, Nili B, Tarro G, Fiazza C and Oleg Yurevich Latyshev
Received: September 28, 2022; Published: October 11, 2022
*Corresponding author: Mauro Luisetto, IMA Academy , Professorship in toxicology , applied and industrial chemistry branch IMA, Italy
DOI: 10.26717/BJSTR.2022.46.007373
Aim of this wor is to verify the property of graphene derivates to increase efficiency in some separation techniques for biomolecule.Various literature report increased effetc due to the peculiar properties of this materials.From laboratory research to the analytical testing in example for m R.N.A. product or other nucleic acid. Of interest also to observe what happen in the large scale biopharmaceutical production ( downstream production of m R.N.A. VACCINE ).This material ( graphene magnetic beads ) showed 170% increase dactivity in separation vs classic methods. But an interesting question : This kind of products are also used in the m R.N.A. vaccine production?. Also siclica resic used in separative column for m R.N.A. purification are classify according their carbon load ( this characteristic can vary in example from 0 to 100% in some provider).And what is the effect on final impurity in graphitic product if used colum whit high % of carbon load?. All this technologies if adopted what kind of toxicological implications can have?.The fact that some independent researcher reported graphene like particle in some vials for covid-19 vaccine need a deeply investigation.
The graphene derivates, quantum dots, fullerene and other carbon producst show particular kind s of properties useful in various settings [1].“ The Carbon materials are universally used in different fields, including in catalysis, adsorption, and sensors . The carbon family includes carbon fiber, carbon dot, graphene, carbon nanotubes, which have diverse sizes, morphologies, and structure porosities . The resultant desirable properties,like, high specific surface area, chemical stability, and excellent electrical conductivity, may also be at the heart of why the carbon materials have recently attracted broad attention . In consideration of the sustainable development of materials, carbon is the second most abundant element in the biosphere next to oxygen. The combination of carbon and hydrogen could offer the basis for a renewable energy source-biomass. Similarly, carbon-based systems play an increasingly important role in emerging renewable energy utilization technologies, including electrodes for energy storage, heterogeneous catalysis, electro- and photo-catalysis, biofuels. The Carbon materials have been widely adopted in gas and water purification. The great potential of carbon materials is also evidenced by some of the highest scientific awards in recent decades, the 1996 Nobel Prize in Chemistry (fullerenes) and the 2008 Kavli Prize in Nanoscience (carbon nanotubes). The development and application of carbonaceous materials is experiencing rapid growth and is a very popular topic in material science “(Figure 1).
In last deecades this products was introduced in various field like biosensors and in other biomedical and biological applications. The chemico- phiscial characteristics of this products make possibile also an use in separation techniques for biopharmaceutical productions.Affinity , or ion exchange chromatography or other ar currently emplyed to purify R.N.A. as reported in many research article . [2]“The increased demand for mR.N.A. vaccines requires a technology platform and cost-effective manufacturing process with a well-defined product characterisation. Large scale production of mR.N.A. vaccines consists in a 1 or 2-step in vitro reaction followed by a purification platform with multiple steps that can include D.N.A.se digestion, precipitation, chromatography or tangential flow filtration TFF. In this review work we describe the current state-of-art of mR.N.A. vaccines, focusing on the challenges and bottle-necks of manufacturing that need to be addressed to turn this newkinf of vaccination technology into an effective, fast and cost-effective response to emerging health crises.
Chromatography is a mainstream purification process widely accepted in pharmaceutical industry. Its high popularity is derived from many attributes like selectively, versatility, scalability and cost-effectiveness . The first published protocol for the large scale purification of synthetically produced R.N.A. oligo-nucleotides used size exclusion chromatography (SEC) in a gravity-flow mode to separate molecules according to size. . Further work studies applying SEC with fast performance liquid chromatography LC were performed . These techniques allowed a preparative- scale purification process, achieving high purity and high yields. SEC presents limitations, as it is not able to remove similar size impurities, like dsD.N.A..The use of ion pair reverse-phase chromatography proved to be an excellent method for the mR.N.A. purification . In IPC, the negatively (-) charged sugar-phosphate backbone of the oligo nucleotides will pair with quaternary ammonium compounds present in the mobile phase (in this case triethylammonium acetate) to become lipophilic and then interact with the stationary phase of a reverse-phase chromatography column . Elution is performed with a gradient of an adequate solvent, like, acetonitrile. Using this approach, dsR.N.A. impurities are effectively removed while maintaining the process’s high yield. IPC is challenging and costly to scale, and the use of toxic reagents like the acetonitrile, is not desirable. A new cellulose-based chromatography process for the removal of dsR.N.A. has been described that leverages the ability of dsR.N.A. to bind to cellulose in presence of ethanol ETOH . This method reported a mR.N.A. yield of greater than 65% with a dsR.N.A. removal of over 90%. Still, the removal of the other impurities was not addressed, and thus the introduction of prepurification steps is likely to be required.
Ion exchange chromatography IEC can also be used to purify the mR.N.A. at large scale. This technique explores the charge difference between the target mR.N.A. species and the different impurities. In example, weak anion exchange chromatography has been successfully implemented to separate mR.N.A. from IVT impurities . IEC presents several advantages: it is scalable and cost-effective; it allows the separation of longer R.N.A. transcripts; and it presents higher binding capacities (when compared with IPC) . This chromatography must be performed under denaturing conditions. This makes the process more complex as it requires a mobile phase heater and a tight control of the temperature during chromatography.Affinity based separation is another mR.N.A. purification approach. A single-stranded sequence of deoxythymidine (dT) - Oligo dT - is routinely used for the capture of mR.N.A. in laboratory applications. This sequence binds to the poly-A tails present in the mR.N.A.. Chromatographic -beads with immobilized oligo dT could thus be used for the process scale purification using affinity chromatography: the poly-A tails of the single stranded mR.N.A. produced during IVT would bind to the stationary phase while impurities are washed out. This way, IVT unconsumed reagents, the D.N.A. template and dsR.N.A. could be efficiently removed. While the high purity products can be obtained using affinity chromatography, several drawbacks are present such as low binding capacities and a less cost-effective process.
The removal of small size impurities can also be achieved while concentrating or diafiltrating solutions by tangential flow filtration (TFF) . Core bead chromatography can also be used for this purpose . In this case, small impurities are trapped inside the beads, and the product will be in the flowthrough. Both techniques rely on D.N.A.se digestion or denaturing agents to remove high size molecules like the D.N.A. template or the polymerase. D.N.A. removal can also be achieved using hydroxyapatite chromatography without the use of a D.N.A.se . As a polishing step, hydrophobic interaction chromatography (HIC) can be applied using connective interaction media monolith (CIM) containing OH or SO3 ligands .
Large scale adaptations of the traditional laboratory scale mR.N.A. purification methods are also being explored. For example, mR.N.A. precipitation can be combined with TFF technique . During TFF, the membrane captures the precipitated mR.N.A. product while other impurities are removed by diafiltration. The product is then eluted by re-solubilizing the mR.N.A.. D.N.A. template removal can be achieved by performing the digestion with immobilised D.N.A.se . Another kind of approach is to use tagged D.N.A. template that can then be removed after IVT using affinity chromatography . Despite being scalable, these methods present a limited effectiveness since they only focus on the removal of some specific impurities and hence must be coupled with other purification steps [3]” “Carbonaceous stationary phases have gained much attention for their peculiar selectivity and robustness. we report the fabrication and application of a graphene-coated polymeric stationary -phase for the anion exchange chromatography.
1. Graphene-coated polymeric particles were fabricated by a facile method.
2. Hyperbranched condensation polymers (HBCPs) were grafted from graphene-coated particles to make anion exchangers.
3. Graphene amount and HBCPs layer count had significant effects on anion exchange capacities.
4. Separation of diverse anionic analytes on the anion exchangers was demonstrated.
5. He prepared anion exchangers exhibited high stability (Figures 2 & 3) [4].
With an observational point of view various relevant literature and references are reported as well website of some producers with technical porperties of their products.Also patents are listed and useful to better understant the focus of this research.Some reference come salso form other field to show the separation charcteristics of graphene derivates.An experimental project hypotesys is submitted in order to provide a global conlusion related the topic investigates.All literature comes from Pub med or other relevant scientific database.
“During the downstream processing, commonly denoted as purification process, the bio-pharmaceutical is purified and isolated to obtain the final product. This is carried out through several purification steps, including filtration, chromatography, tangential flow filtration. In the finishing operation, the final product is placed in its delivery container . The entire process is strictly regulated by FDA, EMA and other international regulatory agencies to guarantee the safety and efficacy of these bio-based drugs . Carbon-Based (Nano) Materials During the last years a great progress has been achieved in the development of new carbon nanomaterials (CNMs) for chemical synthesis, drug delivery, and adsorption/desorption of (bio-)molecules with improved performance .
Carbon nanotubes (CNTs) have been investigated in separation approaches applied to the pharmaceutical and bio medical sectors, mainly due to their strong ability to interact with various bio- (molecules). CNTs are cylindrical sheets formed by a hexagonal arrangement of sp2 carbon-carbon bonds in nanoscale dimensions. The bio compatibility of 3D graphene enables its use as a newgeneration of materials for the purification of pharmaceuticals Magnetic Particles and Other (Nano) Materials Separation using magnetic particles offers several advantages over chromatographic techniques, like the possibility of faster separation in a lower number of steps, reduction of the protein degradation, low costs, scalability and chemical compatibility with ligands already used in derivatized silicas employed in chromatographic systems. The use of functionalized magnetic particles is now regarded as a true alternative for the separation of biologics from complex mixtures . This methodology has been applied in the separation of cells, proteins, peptides, nucleic acids, and pathogens . For many applications, magnetic separation remains as a lab. scale process, though the prospects are for its growing use [5] (Figure 4).”
Figure 4:
(A) SEM of
(i) Aminated silica-coated magnetic beads and
(ii) Graphene oxide modified magnetic beads.
(B) Cross-sectional TEM images of one particle.
(i) Magnetic bead was coated with silica, followed by-functionalizing with amino groups by incubating with (3-aminopropyl) triethoxysilane.
(ii) Graphene oxide was activated with EDC/NHS and conjugated with the aminated silica-coated magnetic bead.
(C) ATR-FTIR spectra of
(i) G.O,
(ii) Aminated silica-coated magnetic beads, and
(iii) Graphene oxide modified magnetic beads, and
(D) Super-magnetic hysteresis loops of graphene oxide GO modified magnetic beads.
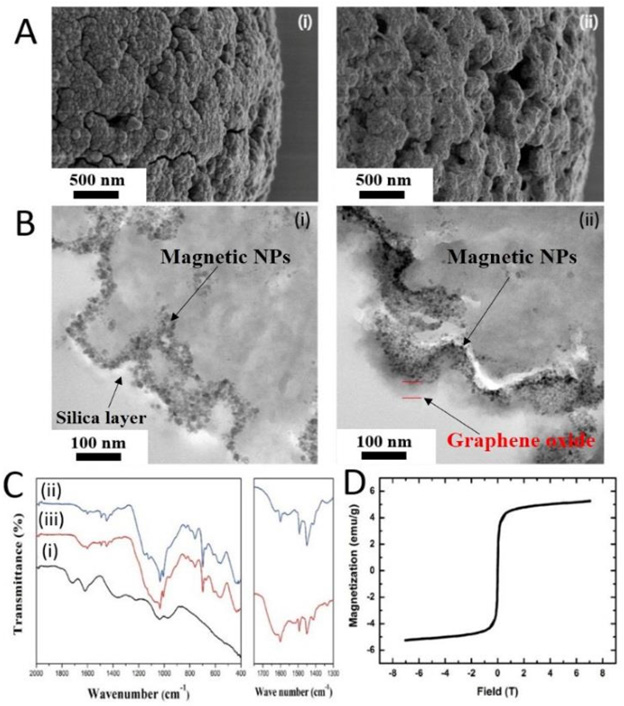
“In this study work , we prepared a streptavidin magnetic bead based on graphene-coated iron nitride magnetic beads (G@ FeN-MB) and tried to use it for the enrichment of severe acute respiratory syndrome coronavirus-2 (S.A.R.S.-CoV-2). The outer shell of our magnetic bead was wrapped with multiple graphene sheets, and there is no report on the application of graphene to the magnetic bead-coating material. First, the graphene shell of G@FeNMB was oxidized by a modified Hummer method so as to generate the carboxyl groups required for the coupling of streptavidin (SA) on the surface of magnetic beads. X-ray photoelectron spectroscopy , Raman spectroscopy, Fourier transform infrared spectroscopy , and transmission electron microscopy (TEM) were used to characterize the oxidized G@FeN-MB (G.O@FeN-MB). Streptavidin was then linked to the surface of the G.O@FeN-MB by coupling the amino of the streptavidin with the carboxyl on the magnetic beads by carbodiimide method; thus, the streptavidin magnetic beads were successfully prepared. To prove the practicality of the SAMBs, biotinylated S.A.R.S.-CoV-2 S1 antibody was linked with it to respectively capture S.A.R.S.-CoV-2 Spike-protein-coupled polystyrene beads (S-PS) and pseudovirus with S-protein expressed. Microplate reader and fluorescence microscope results show that the SAMBs can effectively enrich viruses. The preparation of SAMBs with G@FeN-MB is feasible and has potential for application in the field of virus enrichment. Graphene, as the shell of the G@FeN-MB that we used in this study work, has recently attracted significant attention in the biological field due to its properties, like its thermal conductivity, mechanical strength, large surface area, and high electron-transport capability There have been many studies on the application of graphene in biology, such as in drug delivery , blood glucose sensors , and gene therapy [6] ”(Figure 5).
“[5]In postgenomic era, understanding of protein-protein interactions by characterizing the structure of the corresponding protein complex is becoming increasingly important. An important problem is that many protein complexes are only stable for a few minutes. Dissociation will occur when using typical, timeconsuming purification methods like tandem affinity purification and multiple chromatographic separations. Therefore, there is an urgent need for a quick and efficient protein-complex purification method for 3D structure characterization. The graphene oxide GO ·streptavidin complex is prepared via a GO·biotin·streptavidin strategy and used for affinity purification. The complex shows a strong biotin recognition capability and an excellent loading capacity. Capturing biotinylated D.N.A., fluorophores and Au nanoparticles on the G.O·streptavidin complexes demonstrates the usefulness of the G.O·streptavidin complex as a docking matrix for affinity purification. G.O. shows a high transparency towards electron beams, making it specifically well suited for direct imaging by electron microscopy. The captured protein complex can be separated via a filtration process or even via ongrid purification and used directly for single-particle analysis via cryo-electron microscopy. The purification, sample preparation, and characterization are rolled into 1 single step “[7,8].A magnetic material that consists of silica-coated magnetic beads conjugated with graphene oxide (G.O) was successfully prepared for facile ribonucleic acid (R.N.A.) extraction. When the G.O-modified magnetic beads were applied to separate the R.N.A. from the lysed cell, the cellular R.N.A.s were readily adsorbed to and readily desorbed from the surface of the G.O-modified magnetic beads by urea. The amount of R.N.A. extracted by the G.O-modified magnetic beads was ≈170 % as much as those of the control extracted by a conventional phenol-based chaotropic solution. These results demonstrate that the facile method of R.N.A. separation by using G.O-modified magnetic beads as an adsorbent is an efficient and simple way to purify intact cellular R.N.A.s and/or microR.N.A. from cell -lysates
Figure 6 Graphene oxide modified magnetic bead, process for preparing the same and process for nucleic acid extraction using the same .From KR101800004B1 Google patent,South Korea
Figure 6: Graphene oxide modified magnetic bead, process for preparing the same and process for nucleic acid extraction using the same. From KR101800004B1 Google patent.
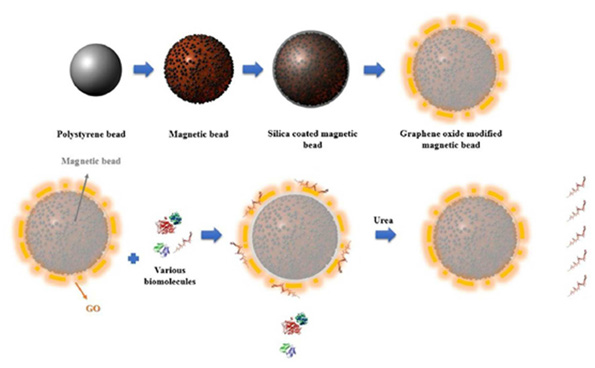
Abstract: The present invention provides magnetic beads modified with graphene oxide, a production method of the magnetic beads, and a separation method for nucleic acids using the same. The magnetic beads modified with oxidized graphene of the present invention can be usefully used for a simple and convenient method for purifying single stranded D.N.A. or R.N.A. in bio-technology where extraction of small molecule R.N.A. like miR.N.A. is essential. “The efficiency of magnetic separation is particularly suitable for mass-scale purification.” Form https:// magnetics.life/. C·Prep Magnetic Beads “R.N.A. extraction, R.N.A. purification, R.N.A. storage, R.N.A. shipping, single stranded D.N.A. and plasmid extraction” . And from https://www.vdobiotech.com/
Description: VDO biotech’s magnetic microsphere for viral nucleic acid is a core-shell structure and polydisperse magnetic microsphere, and the surface is coated with silicon hydroxyl groups. MS02HA has fast magnetic response speed, excellent suspension, and outstanding hydrophilicity and nucleic acid capture ability. MS02HA is highly efficient in extracting nucleic acids from virus, pseudovirus particles and small fragments. It is suitable for a variety of sample types, suitable for automated nucleic acid extraction, and is an ideal choice for biological sample purification.
Advantages:
1. Large specific surface area: the high capability to combine with nucleic acid
2. High hydrophilic property: reduce the possibility of the beads adhering to the tube walls
3. Multiple sample compatibility: are suitable for the virus, pseudoviral and small nucleic acid fragment
4. Special chemical groups: the high capacity to adsorb nucleic acid and ease to elute.
(From http://www.szbknano.com/bocpen.php?id=109).
“Suzhou Beike Nano Technology Co., Ltd. improves the quality of magnetic graphene. Magnetic graphene is composed of reduced graphene oxide loaded with magnetic Fe3O4 nanoparticles. It has magnetic responsiveness, catalytic properties, and graphene’s electrical and thermal conductivity properties. it can be used for magnetic separation of biomolecules, as a simulated enzyme for biocatalysis, and environmental protection. Contamination treatment, doped polymers, etc. to construct composite materials, construct nano-medicine carriers, develop biosensing and detection methods, etc.”
(From https://magnetics.life/news/R.N.A.-news/R.N.A.- purification/) “R.N.A. is far more fragile than D.N.A. – while the halflife of D.N.A. is 531 years, the half-life of R.N.A. is just 30 minutes. This means that working with R.N.A. is far more difficult in general, but it’s also considered to be the next revolution in life sciences. Developing a R.N.A. purification product will be Life Magnetics’ first step to proving the technology works and establishing a viable revenue grounding for the product, after which diagnostic testing or synthetic R.N.A. manufacturing is made possible”.
Of Interest a Discussion with a Producers of Graphene Products for Separation:
Mauro Luisetto < maurolu65@gmail.com >
mar 20 set
dear org do you provide GRAPHENE MAGNETIC BEADS for R.N.A. purification large scale volume
industrial vaccine m R.N.A. production ?
Producer REPLAY : K. H. 17:50
Yes, we can do high volume extractions.
The carbon beads will bind all R.N.A. under the following conditions:
100 mM CaCl2 present
Either a) 50% ethanol or b) 90 C heating
If the R.N.A. is short and does not have secondary structure such as miR.N.A. or R.N.A. oligos, then the second step with the ethanol or heating is not required. Only the 100 mM CaCl2 is required.
Mauro Luisetto < maurolu65@gmail.com >
19:03 to K. Producers :
Please can you send me scientific literature about use in production-large scale
R.N.A. purification?
whit graphene coated magnetic beads the separation increase in efficiency .
you provide also graphene coated beads for affinity or ion chromatographic separation column?
regards
producer replay :
Yes,
Here’s an overview of some example successful implementations and what conditions are necessary depending on the length of the nucleic acids your trying to isolate.We only have magnetic beads currently. We’re still a startup.This format works well for large volumes. Our first applications have been in urine and wastewater surveillance. The carbon is structurally selective. The nucleic acids need to be linear and unfolded to bind. What nucleic acids bind depend on how complex the structure is, but this can be controlled. The nucleic acids will denature with heat like you do in PCR, PCR uses 95 C to melt D.N.A. into single strands. Same thing will work here. Ethanol can also be used, it serves the same function.
As far as I know, we are the only commercial producer of graphene magnetic beads for this application and it is not currently being used for vaccine manufacturing.We are collaborating with a company who is doing pharmaceutical manufacturing, they are making a colorectal cancer drug though and not a vaccine. In this case, it is actually being used for both separation and delivery. Immediately after the separation, they feed the beads with R.N.A. bound to mice and they have seen evidence this is effective for drug delivery.Inside of cells, the Ca2+ concentration drops as compared to outside the cells. When the R.N.A. and carbon bound together enter the cells, the R.N.A. is released and makes protein.So, there are many kinds of carbon. These mostly focus on graphitic oxide. We do not make graphitic oxide. The form of carbon we make is highly reduced. So what we use is not the same.I am skeptical of the claim that they see 170% improved yield. Our experience is that most kits give 100% yield. We have had Qiagen, Promega, and BioRad all perform comparisons and the result has been that all kits provide the same yield within 5-10%.
There are many different mechanisms by which binding can take place. On silica magnetic beads electrostatic interaction is the dominant mechanism. One paper uses urea. Urea will not work with our materials. It’s a different carbon. Another paper uses conjugation. Conjugation is the same on silica as on carbon, there is no reason to specifically use carbon in that case.The advantage of our carbon specifically is that it operates with Ca2+ as the binding and release mechanism. This is scalable to large volumes. It’s easy to use a 4L solution with just 100mM Ca2+. The processes proposed here use more complex lysis buffers. This can be a problem in large volumes because if you need for example 3M GITC, that is an aquatic toxicant and waste disposal is problematic. The carbon beads, the Ca2+ and the EDTA are all environmentally friendly and cheap so scale up is easy. Also, it is structurally specific, so you can separate short from long R.N.A. for example. This is something graphitic oxide can’t do.
I realize there are many people researching carbon. We make a very specific type of carbon for a specific purpose and carbons are not interchangeable.We have sent samples to and collaborated with Qiagen, Promega, and BioRad. With cell cultures, all commercial methods get 100% yield. It is therefore not possible to get 170%. The only explanation for this would be if they performed the standard extraction improperly in the first place. For example, if they were to perform an extraction poorly such that R.N.A. recovery was only 30% and then they did an extraction with their G.O and the yield was 45%, then this would be a 175% improvement. The reality is they really just did a poor extraction with the standard method. Our carbon is coated onto magnetic beads conformally. Again, it is also not G.O.
Other question to the producer :
Do you know what technologies used in purifying M R.N.A. covid vaccine ?
producer replay :
They use TFF filtration for separation of D.N.A. and R.N.A.. Some provider are currently evaluating our technology and they supply producers of m R.N.A. VACCINE .
See https://www.pall.com/en/laboratory/tangential-flowfiltration. html
TFF filtration can separate R.N.A. from D.N.A.. The process is inefficient. The limitations are that it cannot separate dsR.N.A. from ssR.N.A.. ds (double stranded) R.N.A. is a contaminant which lowers the efficacy of the vaccine. So there is a secondary column filtration to remove dsR.N.A.. Also, the TFF filters need to be replaced often. Over time, the TFF filters become «lubricated» with lipids and D.N.A. contamination starts to slip though.We are working with them on a better process where the carbon we make pulls off only the R.N.A. and leaves the D.N.A. behind using Ca2+ as the binding agent. The translation reaction can happen in the presence of 100 mM Ca2+ so this would allow R.N.A. to continuously be removed in the process. The magnetic beads are then isolated and the R.N.A. released (Figures 7 & 8).
Other question to the producer : i ask also the secondary columns what resin use?and the TFF filters?
this use carbon , graphene, fullerene or quantum dots ?
Producer replay :
Polysulfone membranes.
There is no resin, it’s a filter. it’s a plastic filter with holes in the walls of a specific size.
Separation of dsR.N.A. and ssR.N.A. is done with silica columns.
Separation of R.N.A. and D.N.A. is done with a polysulfone size filter, but the polysulfone is not reactive in any way, it’s simply a plastic with holes of a specific size.
Other producer :
Dear Dr. Luisetto,
I think graphene is not necessary to your purpose, our regular magnetic bead and resins can purify mR.N.A. very well.
Currently we do not produce graphene product. If you need us to customize it for you, pls provide detail data about the graphene product you need.
Affinity monolithic column based on graphene-nanogold composite interface ultrahigh-load aptamer and preparation method there of.
Abstract: “The invention belongs to the field of preparation of monolithic column polymeric materials, and particularly relates to an affinity monolithic column based on a graphene-nanogold composite interface and an ultra-high nucleic acid aptamer and a preparation method thereof. The method comprises the steps of preparing a graphene functionalized hybrid silica gel polymeric monolithic column by a one-pot method by using surface-modified graphene oxide as a catalyst and a modification material, modifying a nanogold column to the surface of a graphene material of the modified silica gel porous monolithic column, and realizing ultrahigh-density loading of an aptamer on the surface of the modified silica gel porous monolithic column based on the bridging action of nanogold. The modified silica gel porous monolithic column matrix has a high specific surface area, the modified graphene oxide on the surface of the modified silica gel porous monolithic column matrix can stably and efficiently load nano gold particles, meanwhile, the agglomeration phenomenon of the graphene and the nano gold particles in the preparation process is avoided, the aptamer can be modified on the monolithic column in a high density manner, and the silica gel hybrid affinity monolithic column can be used for efficient specific recognition and separation of ochratoxin A.
Description: Affinity monolithic column based on graphenenanogold composite interface ultrahigh-load aptamer and preparation method thereof.
Technical Field: The invention belongs to the field of preparation of monolithic column polymeric materials, and particularly relates to an affinity monolithic column based on a graphene-nanogold composite interface and an ultra-high nucleic acid aptamer and a preparation method thereof.
Background: The aptamer is a short-chain D.N.A. or R.N.A. sequence screened from a large-capacity random oligonucleotide library by an in vitro screening technology (SELEX), can be combined with a corresponding target ligand in a high-affinity and high-specificity manner, and has the advantages of wide applicable target range, easiness in synthesis and modification, stable chemical properties, high specificity and the like”.Aptamers are short sequences of artificial D.N.A. or R.N.A. that bind a specific target molecule.
Patent: Graphene Media For Chromatography, Application WO- 2016057715-A1.
Abstract: “Described herein are methods separating mixtures of organic materials, the methods comprising retaining a graphene oxide; contacting the mixture with a graphene oxide; passing an organic eluent over the graphene oxide to concurrently elute the organic materials in the mixture at different rates; and selectively collecting the at least one organic material from the passed eluent, separating the at least one organic material from another. Also described herein are chromatographic materials, columns and systems incorporated in same methods” [9].“In the last decades, the separation technologies have been significantly furthered by the development of a variety of new separation media. Especially, the carbon-based nanomaterials (CNMs), including graphene, carbon nanotubes, fullerenes, have been applied for effective separations and sensitive detections in recent years. Here, the fundamental preparation protocols of new separation media consisting of CNMs and a great number of their applications summarize the fundamental preparation protocols of new separation media consisting of CNMs and a great number of their applications are here summarized. Graphene and Carbon Nanotubes.
As well as SPE and GC, graphene-based stationary phases for LC have been aggressively studied. Li et al. developed a porous polymer monolithic column by copolymerization of a conjugated G.O with-(trimethoxysilyl) -propylmethacrylate and ethylene dimethacrylate. Liang et al. achieved the efficient separation of various phenols by G.O modified silica particles (G.O-Si). The GOSi showed high resolution for PAHs due to the π-π interaction and separation of polar analytes, such as hydroxybenzenes and amine compounds, by the functional groups of G.O. Additionally, they improved the performance of the G.O-Si by the modification with O.D.S. to suppress the effect of polar functional groups, resulting in higher separation efficiency for a variety of aromatic compounds. Zhang et al. also improved the G.O-Si by treatment with hydrazine to reduce the functional groups, then the prepared column indicated a stronger retention of aromatic compounds.53CNTs have also been applied to stationary phases in L.C. Aral et al. developed a hybrid material with carboxy-induced SWCNT and amino-Si. The prepared column showed remarkable separation efficiency (plate number of 25000 on 150 × 4.6 mm column) and effective separation of aromatic compounds. In particular to SWCNT, it has a chirality based on the right or left hand coil. Ahmed et al. revealed the possibility of enantio-selective separation of the racemic medicines using an SWCNT-introduced silica monolith. SWCNT stationary phase is also useful for peptide research”[9].
And Related Other Field : to Show Interaction Between Carbon Nanotubes and D.N.A: “D.N.A.-based Ion Exchange Chromatography, This separation method is based on D.N.A.-wrapped SWNTs (Singlewalled carbon nanotubes (SWNTs), showing different electrostatic interactions with an ion exchange (I.E.X.) column. A stable barrel is formed around nanotubes with 2D H-bonding with single-stranded D.N.A. (ssD.N.A.) wrapped helically around individual CNTs , exposing the deoxyribose phosphate. The selection of nanotubes is believed to originate from the electrostatic and electrodynamic interactions between the D.N.A. barrel-nanotubes and the ion exchange resin. Pure chiral nano-tubes were separated by this method for the first time. A recent experiment showed the improved structural motive of ssD.N.A. allows for separation of 12 different chiral CNTs with 60-90% purity .The estimated yield of chiral (n, m) tubes is very low; the majority of nano-tubes are expected to be adsorbed onto the ion exchange column in a bundled state. A major drawback of SWNT separation by D.N.A.-based ion exchange chromatography IEC is the high cost of ssD.N.A. used and expensive manufacturing process (high cost of the IEX column and its unavoidable replacement caused by clogging)”[10].“Biomolecules that can interact with graphene include proteins and nucleotides (D.N.A./R.N.A.), Vashist and Luong (2015) studied target proteins and found that nucleotides D.N.A./R.N.A. were highly adsorbed onto the surface of electro chemically and thermo chemically reduced Rg.O. Compared with the surfactant dispersion system, although the concentration of graphene was much lower in the bio-molecular system than in the surface active system“[11] (Figures 9 & 10].
Figure 9: Scheme of physical interactions between column and target molecules, based on
(A) Affinity,
(B) Electrostatic forces, and
(C) Size difference in liquid chromatography LC systems.
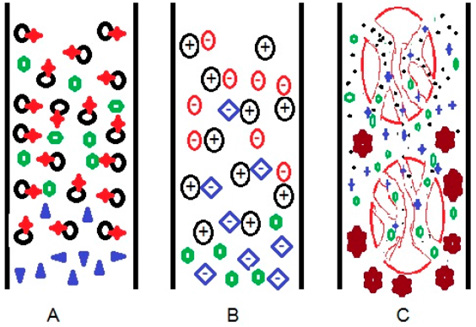
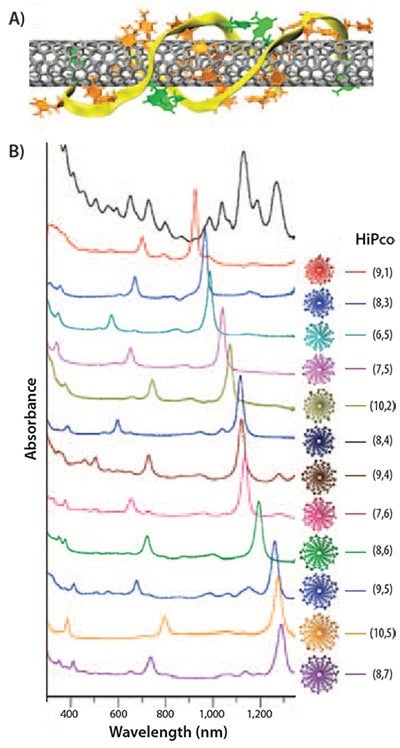
Figure 10: Separation of SWNTs using single-stranded D.N.A. ribbons.
A) Anti-parallel D.N.A. (ATTTATTTATTT) strands wrap around (8,4) SWNT held by hydrogen bonding between D.N.A. strands and ππ interaction between D.N.A. and SWNTs.
B) UV-Vis-NIR absorption spectra of 12 purified SWNTs with different chiralities separated by ion exchange column IEC chromatography. Sorting of each SWNT can be enhanced by using different D.N.A. sequences.
Nano-Carbon Material: Nanocarbon materials (e.g. carbon nanotubes, graphene, fullerene) exhibit very interesting features. Based on their unique chemical structures, nanocarbon materials exhibit conductive properties and are used in applications such as: high-speed transistors, photovoltaics, sensors, etc. These materials represent the future of materials science and microelectronics. Advanced nanocarbon reagents are available. For research use or further manufacturing use only. Not for use in diagnostic procedures.“Carbon dots (CD) are also able to provide better separation efficiency than commercial columns for the equivalent C18 type. In affinity-based separation , stationary particles (black) with specific functional groups (red) interact with the appropriate compounds (green) through specific binding via a network of interactions, resulting in retention in the column. The stationary phase does not suspend noninteracting compounds (purple), and the compounds flow without retention. The common interactions in this mode of separation are hydrogen bonding, dipole–dipoles interaction, London force, and complex formation. The combination of hydrogen and dipole–dipole interaction facilitates a hydrophilic interaction, while the London force is a hydrophobic interaction. Complex formation commonly occurs in chromatography where the analytes are proteins , D.N.A. , and R.N.A.”[12] (Figure 11).“Nucleic acids are relevant biopolymers in therapy and diagnosis, for which their purity and biological activity are of crucial relevance” [13] (Figure 12).
Ultrafiltration preserves biological activity and saves time - Protein purification technology has progressed from methods as diverse as chemical precipitation for the sample concentration or dialysis for buffer exchange towards pressure-driven purification cross flow systems utilizing ultrafiltration membranes. Ultrafiltration (UF) techniques rely on the use of polymeric membranes with highly defined pore sizes to separate molecules according to size. Simply put, UF procedures rely on the use of fluid pressure to drive the migration of the smaller molecules through an UF membrane with the simultaneous retention of larger molecules. While chemical -precipitation can be used to concentrate a protein sample, separation with ultrafiltration is based on mechanical rather than chemical interactions allowing a researcher to perform sample concentration without the addition of denaturing solvents or salts. Buffer exchange using dialysis technologies use large volumes of buffer and since the only force acting upon the solution is diffusion, the process can take several days. Pre-assembled and simple to use ultrafiltration devices can rapidly perform either concentration or buffer exchange procedures without the extensive handling required for many other techniques.Ultrafiltration can be performed in one of 2 operational modes: Direct Flow Filtration , or Tangential Flow Filtration (TFF). DFF works well for the small volumes (up to 30 mL) using centrifugal devices, DFF technologies can fall prey to problems with membrane fouling. To reduce the formation of a gel layer, cross flow can be generated on the upstream side of the membrane using a floating stir bar configuration (stirred cell) or by creating a controlled laminar flow. While stirred cell operations tend to improve UF performance, they are still limited to achieving the optimal performance since the velocity and subsequent level of agitation is dependent on the sweep of the bar that varies along the radius of the sweep (Figure 13).
TFF is a rapid and efficient method for separation and purification of biomolecules. It can be applied to a wide range of biological fields like immunology, protein chemistry, molecular biology, bio-chemistry, and microbiology. TFF can be used to concentrate and desalt sample solutions ranging in volume from 10 mL to thousands of liters. It can be used to fractionate large from small biomolecules, harvest cell suspensions, and clarify fermentation broths and cell lysates.
Easy to set up and use – Simply connect the TFF device to a pump and pressure gauge(s) with tubing and a few fittings, add your sample to the reservoir, and begin filtration.Fast and efficient – It is easier to set up and much faster than dialysis. Higher concentrations can be achieved in less time than when using centrifugal devices or stirred cells.Perform 2 steps with one system – Concentrate and diafilter a sample on the same system, saving time and avoiding the product loss.Can be scaled up or scaled down – Materials of construction and cassette path length allow conditions established during pilot-scale trials to be applied to process scale applications. TFF devices that can process sample volumes as small as 10 mL or as large as thousands of liters are available.Economical – TFF devices and cassettes can be cleaned and reused, or disposed of after single use. A simple integrity test can be performed to confirm that membrane and seals are intact. Key Applications for TFF.
The primary applications for TFF are concentration, diafiltration (desalting and buffer exchange), and fractionation of large from small biomolecules. In addition, it can be used for clarification and removal of cells, as well as cellular debris from fermentation or cell culture broths”.
Abstract: To develop a high-performance thin-film nanocomposite (TFN) nanofiltration (NF) membrane, the functionalized graphene-based nanomaterial was synthesized by combining GO. Hyperbranched polymer, which has been used as a novel nanofiller and successfully integrated into the active polysulfone (PSf) layers through an interfacial polymerization (IP) process.
Fourier Transform: Infrared Spectroscopy (FTIR) and Fieldemission Scanning Electron Microscope (FESEM) were used to characterize the NF membrane obtained and its performance was evaluated according to the water flux with the addition of f-GO, TFN0.05 membrane exhibit the optimal, and salt rejection rate. The influence of f-G.O on the morphologies, properties, and performance of TFN NF membranes was investigated.The addition of the f-G.O membrane exhibited the optimal water flux without the sacrifice of the salt rejection. It was found that the introduction of functionalized graphene-based nanomaterial nanosheets favored the formation of a thinner and smoother nanocomposite active layer with enhanced hydrophilicity. As a result, TFN NF membranes demonstrated a superior permease activity over the conventional thinfilm composite (TFC) membranes [14].” “The mR.N.A. was purified by oligo-dT affinity purification, buffer exchanged by tangential flow filtration into sodium acetate, pH 5.0, sterile filtered, and kept frozen at –20 °C until further use” [10].“Commercially available magnetic particles that are suited for nucleic acid separation can be obtained from a variety of companies [15]. Mostly, the matrixes are based on silica, porous glass, cellulose, agarose, polystyrene and silane. Some important patents exist that describe synthesis of magnetic carriers not only for nucleic separation”[16].
Get in the mR.N.A. Vaccine Race with Affinity Purification: “With demand for the large-scale production of clinical-grade mR.N.A. suddenly surging, developers need fast, efficient, and highly scalable methods for mR.N.A. purification. The bench-scale mR.N.A. purification methods used until now are becoming a significant bottleneck to large-scale manufacture. Thermo Fisher Scientific has developed a new affinity-based mR.N.A. purification product, Thermo Scientific™ POROS™ Oligo (dT)25 affinity resin, tailormade for scalability. The mR.N.A. binds selectively to the surface of the Oligo (dT) beads, and any impurities are simply washed away. As mR.N.A. can be rapidly manufactured in a cell-free system, mR.N.A. vaccines are faster to produce than protein vaccines. The switch to affinity purification-already used to purify protein-based biologic drugs at large scale – should further streamline the process. here are several other reasons mR.N.A. lends itself to vaccination, Kowalski says. Protein-based vaccines generally require a second ingredient, known as an adjuvant or excipient, to boost the immune response and help ensure the antigen generates strong, longlasting immunity. With mR.N.A., The immune system is inherently on the lookout for it because many viruses are R.N.A.-based. “The mR.N.A. is a natural adjuvant, which helps to boost the immune response,” Kowalski says. The body’s virus surveillance systems can be exploited in further ways to boost mR.N.A. vaccine efficacy, says Harry Al-Wassiti, who is developing mR.N.A.-based vaccines and therapeutics at Monash University. He and his colleagues have been researching the lipid nanoparticles used to encapsulate mR.N.A. prior to injection. “The nanoparticles have 2 roles: to protect the mR.N.A. and to deliver it to cells,” Al-Wassiti says. For vaccines, immune cells called antigen-presenting cells (APCs) must see the antigen to elicit the immune response.
“From the surface, these lipid nanoparticles look almost like a virus, so the APCs take them up,” he says. The Monash team and others have been developing lipid nanoparticles that APCs recognize particularly effectively. Another advantage of mR.N.A. therapeutics compared with proteins is that, from a purification standpoint, all mR.N.A. molecules are essentially identical, Al-Wassiti says. That’s because the amino acids in proteins are chemically diverse, while the ribonucleic acids that make up mR.N.A. are relatively similar. “Whereas protein purification really varies depending on the protein sequence, all mR.N.A. looks the same, so the way you purify, it is the same,”Al-Wassiti says. It’s therefore likely that large-scale mR.N.A. affinity purification protocols pioneered for vaccine development could be readily adopted for mR.N.A. therapeutics. When Thermo Fisher approached AmpTec in 2019 to test a new affinity-based mR.N.A. purification product specifically designed for large-scale applications, the company was very receptive to the idea, Scheinert says. “We were very excited, because it is very important for us to actively prepare for the increasing requests and demands from the market regarding scale,” he says. “mR.N.A. production scales will certainly increase, and we need purification options available that can deal with large scales. Thermo Fisher customers had been requesting custom solutions for large-scale mR.N.A. purification, according to Scott Zobbi, the firm’s business lead for custom POROS chromatography resins. “When 3 or 4 people start to ask you the same thing, you realize there’s a broader demand,” he says. Kelly Flook, its senior product manager for bioprocess purification resins, led the development of an mR.N.A. affinity purification product that would be available to all customers: POROS Oligo (dT)25 affinity resin. AmpTec is among several mR.N.A. manufacturing companies that have helped put the new product through its paces. Oligo (dT)25 resin leverages the fact that all mR.N.A. molecules, natural and synthetic, feature a poly-A tail, a stability-enhancing chain of adenine nucleotides at one end of the molecule. The product, which exploits complementary base pairing between adenine and thymine to isolate mR.N.A. after synthesis, consists of porous polymer beads coated with deoxythymine (dT) strands that can capture mR.N.A.’s poly-A tail. At the end of an mR.N.A. synthesis, the reaction mixture is combined with a sodium chloride solution, then loaded onto a column filled with Oligo (dT) beads. The sodium ions in the salt solution neutralize the negative charges found along the backbone of the R.N.A. molecules; that allows the poly-A tail to form hydrogen bonds with the dT strands on the beads. The impurities from the reaction mixture are simply washed off the column when flushed with more of the salt solution. When the column is flushed with fresh water, the sodium ions are then washed away. The negative charges on the backbones of the Oligo (dT) and the poly-A tail repel each other, breaking the base pairing and releasing the now purified mR.N.A.. “Within a couple of column volumes, you will have collected a purified, concentrated solution of your target mR.N.A.,” Flook says. Part of the product’s appeal is that dA-dT affinity binding is a tried and true method for purifying mR.N.A. samples at bench scale, Scheinert says. The new product transfers the Oligo (dT) coating to a 50 μm polystyrene divinyl benzene cross-linked porous bead. Compared with typical HPLC resins, the bead is large, according to Zobbi. “But because it has inherent porosity, you have an increased surface area,” Zobbi adds, which means greater capacity to bind mR.N.A.. And as it’s a bead-based product, users have the flexibility to pack it into a column of any size. They can tailor the purification step to the scale of the mR.N.A. sample to be purified.
Basing Oligo (dT) on the proved POROS bead technology inspires confidence in the product, says Joseph Barberio, the director of process development at Strand Therapeutics, a seedstage biotech company developing programmable mR.N.A. therapeutics based in Cambridge, Massachusetts. “POROS resins are proven at scales from benchtop to commercial manufacturing operations,”says Barberio, who has tested the new resin. “Utilizing the same technology from early development through scaled manufacturing is key to the successful tech transfer and execution of a manufacturing campaign.”Barberio says his experience with Oligo (dT)25 resin has so far been positive.“For so long, the R.N.A. sector has been working with resins that were not made for R.N.A.. It is really great to see a major manufacturer focused on designing products for the mR.N.A. space.”[17].
Thermo Scientific™ Hypercarb™ Porous Graphitic Carbon HPLC Columns.
Catalog number: 35005-151030 Related applications: Chromatography.
“Offer 100% porous graphitic carbon for extended separation capabilities with Thermo Scientific™ Hypercarb™ Porous Graphitic Carbon LC Columns. Enhanced retention of polar compounds and separation of structurally related analytes with stability at pH extremes and high temperature.”
Thermo Scientific™
D.N.A.Pac™ PA200 Oligonucleotide HPLC Columns Catalog number: 062998
Related applications: Chromatography
Enjoy strong anion exchange for high-resolution analysis and purification of synthetic oligonucleotides with Thermo Scientific™ D.N.A.Pac™ PA200 Oligonucleotide HPLC Columns.“The Thermo Scientific™ D.N.A.Pac™ is available in either RP or IEX mode for separation of oligonucleotides and double stranded D.N.A./R.N.A. fragments”. Does Carbon Load Affect the Retention Times from Your https://www.chromatographytoday.com
“30 Aug 2018-Carbon load refers to the percentage carbon content of the silica in the stationary phase-the silica bonded to the inner walls of the column.”In example % carbon load of various type of column fron an international provider vary from 0-100 %.Carbon load is expressed as the percentage of carbon per weight of silica.Carbon load refers to the % (by weight) carbon content of the stationary phase bonded to the support material”. (Figures 14- 16).
Figure 14: Schematic diagram of silica-carbon composite material. Carrier is silica gel, and carbon layer is high graphitization degree.
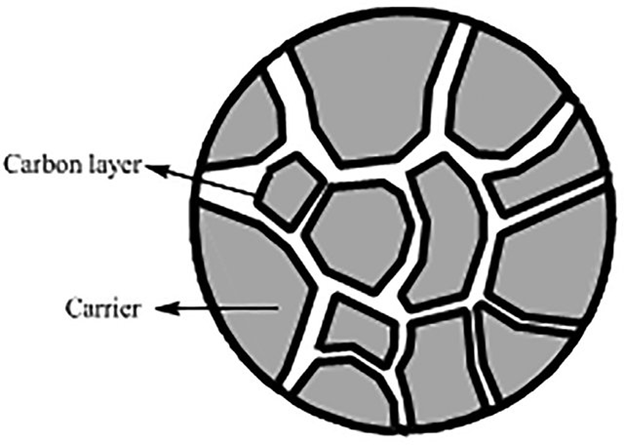
Figure 15: For Other kind of separation (G.C) Carbon silica gel has a structure in which activated carbon is deposited on the surface of spherical silica gel. By controlling the amount of the activated carbon on the surface, it is possible to prepare carbon silica gel with different adsorption forces according to the GC/MS measurement conditions. 3 different types of carbon are available. 2-layer cartridge columns are filled with 2 types of carbon silica gel with different adsorption properties, which is improved by the conventional cleanup method using a single-layer activated carbon silica gel column.
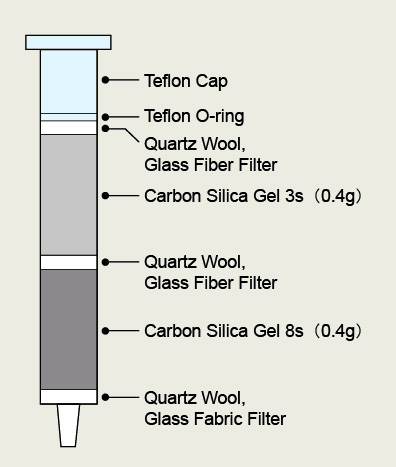
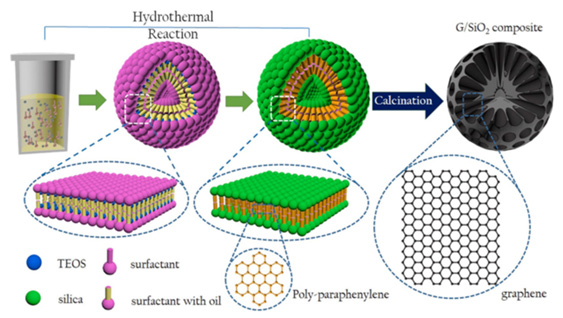
Figure 16: Schematic diagram of graphene/silica growth mechanism. Reprinted/adapted with permission.
Large scale preparation of single chitin oligomers by the combination of homogeneous acid hydrolysis and reversed phase preparative chromatography. “The silica-carbon composite material showed high graphitization degree, controlled morphology and pore size, which could provide strong retention of highly polar compounds in reversed phase chromatography RPC mode, especially for carbohydrates . In this study work, the silica-carbon composite material was employed as the packing of preparative chromatographic column to separate chitin oligomers, and five highly purified single chitin oligomers were obtained at gram scale” [17]. Graphitic mesoporous carbon-silica composites from low-value sugarcane by-products for the removal of toxic dyes from wastewaters. “Highly porous carbon-silica composites (C.S.C) were prepared for the first time through a simple wet impregnation process and subsequent pyrolysis of low-value sugarcane byproducts, namely molasses. These C.S.C materials demonstrate a distinct range of functionalities, which significantly differ from similar materials published in literature. The carbon-silica composites prepared at 800°C exhibited exceptional adsorption capacities for the azo-dye congo red (445 mg g−1), due to the kind of graphitic carbon coating and unique functionality including C-O-C within the porous structure. Congo red adsorption capacity of the highly mesoporous graphitic carbon-silica composites significantly exceeds that of commercial activated carbon and silica, these carbon-silica composites represent an effective step towards the development of porous bio-derived adsorbent for remediation of dye waste-waters. Both the porous properties (surface area and pore size distribution) and the functionality of carbon coating were dependent on the temperature of preparation” [18].
“Separation is 1 of the most important unit operations in chemical engineering. In order to prepare a stationary phase with hydrophilic interaction for liquid chromatography, Zhao et al. used silica micro-spheres as a carrier and cyclodextrin as a carbon source to prepare a carbon-coated composite by the hydrothermal carbonization. Cyclodextrin and the polyvinylpyrrolidone were first added to a Teflon liner containing deionized water along with silica microspheres. After the hydro thermal reaction, the slurry-packed capillary columns containing the carbon-silica stationary phase exhibited excellent chromatographic repeatability, separation selectivity, and pH stability for the polar compounds, like phenols and endocrine disrupting chemicals . Since the trade-off between the polarity and selectivity, especially for polar organics, is a common issue in adsorption, Yang et al. used waste lithium–silicon powder and commercial activated carbon as resources for preparing a zeolite-activated carbon composite material, so as to combine the advantages of activated carbon and molecular sieve” [1].
In order to verify the possibility in contamination with carbon products of purified m R.N.A. it is interesting to test the final product using for the purification :
1. Silica resin with very low carbon load and
2. Silica resin wiht high carbon load.
Results
If there is a significative difference ( > 0,05 insignificative way) of carbon -grafitic produtcs on this final m R.N.A. PRODUCT it showed that the carbon load of a separative silica colum can affect the final material.
In the review part of this work are showed the properties of graphene magnetic beads and efficiency is purification of biomolecule.This products are used in research laboratory and in analytical process for extracion of m R.N.A. in samples.The m R.N.A. vaccine production need purification phases and are used often various chromatographic separation ( affinity , ion exachenge and other ). Magnetic separation was introduced since 1970 years , and various manifacturers produced this magnetic beads .Mgnetic beads are used in purification of biopharmaceuticals and inside this class of products. Is possible to see normal magnetic beads but also coated ( in example graphene shell magentic beads ).But if this are currently in use in small laboratory scale to enrich sample to be tested what happen in large scale production ? ( in example in dowstream process)?.On the other hand : separation colum used for m R.N.A. purification are silica resin. In the commerce there are various kind of this resin resin with different characteristics : one of this is related the Carbon Load ( grafitic).
Due by intrinsic chemico -phyical properties of graphene derivates that make possible to increase efficiency in m R.N.A. purification it is needed to be verified:
1. The role played in large scale production
2. The related toxicological profile
(if there is the risk that impurities of graphene can be finded in the final products.)The same it is needed to know if and what kind of resins are used (silica?) and the related carbon load.( graphitic). In commerce there are various kinds of this resins and with a carbon load that vary from 0% to 100%.For this reason every m R.N.A. VACCINE producers must declare what purification process used and what material employed .The regulatory agency must to have full possibility to verify this condition.


