Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Min-ya Lu1,2*, Li-song Teng2, Zhe Wang3, Xiao-dong Teng4 and Zhao-Ming Wang4
Received: September 05, 2022; Published: October 11, 2022
*Corresponding author: Min-ya Lu, Department of Pathology, The Affiliated Hospital of Hangzhou Normal University, Hangzhou 310000, Zhejiang Province, China and Cancer Center, The First Affiliated Hospital, Zhejiang University, Hangzhou 310000, Zhejiang Province, China
DOI: 10.26717/BJSTR.2022.46.007370
Background: Large B-cell lymphoma (LBCL) with interferon regulatory factor 4 (IRF4) rearrangement (LBCL-IRF4) is a rare entity of LBCL with a specific clinical presentation. Notably, it is defined by the presence of an IRF4 rearrangement.
Case Presentation: Here, we describe a case of a 2-years-old child presenting with a LBCL-IRF4 located in the left tonsil, with characteristic histologic appearance and the phenotype of neoplastic cells. The histologic analysis showed the monomorphic atypical lymphoid cells were medium size or large, positive for CD20, CD79a, MUM-1, GATA3, and Ki-67 was about 80%; Fluorescence in situ hybridization (FISH) analysis confirmed the presence of an IRF4 rearrangement. The patient was followed up for 18 months with no evidence of recurrence after being treated with 1 cycles of R-CHOP.
Conclusion: Morphologically, LBCL-IRF4 has predominantly diffuse or follicular pattern with a large number of necrosis, and a high proliferation rate, all of these suggest that the tumor is highly invasive. But actually LBCL-IRF4 usually performs a favorable outcome after therapy. Furthermore, LBCL-IRF4 with GATA3 positive have never been reported
Keywords: Large B-cell Lymphoma; IRF4; MUM1; GATA3; Ki-67
Abbreviations: DLBCL: Diffuse Large B-Cell Lymphoma; FISH: Fluorescence In Situ Hybridization; FL: Follicular Lymphoma; LBCL: Large B-cell Lymphoma; IHC: Immunohistochemistry; IRF4: Interferon Regulatory Factor 4; LBCL-IRF4, Large B-cell Lymphoma with IRF4 Rearrangement; ISH: In Situ Hybridization
IRF4 encodes for the IRF4 protein belonging to the IRF family of transcription factors controlling some important links in B lymphocytes proliferation and differentiation, including pre-B cell differentiation, marginal B cell development, gerogenic center reaction and plasma cell differentiation. Therefore, the expression of IRF4 is involved in the transformation of some lymphoid proliferating diseases [1-3]. IRF4 is described as a multipartner gene, including IGH, IGL, and IGK. Salaverria , et al. [4] identified the different types of IRF4 rearrangements. LBCL-IRF4 has recently been included in the World Health Organization (WHO) classification. L. de Leval and C. Bonnet, et al. [5] analyzed most LBCL-IRF4 occurs in the Waldeyer ring, and most patients have isolated lymph node enlargement in the head and neck, or tonsillar enlargement (clinical stage is l-II stage). It occurs mainly in children and young people, men are more common, but the reason is unknown [6,7]. The characteristic change is the tumor cells strong express the immunohistochemical of IRF4/MUM1. Resent research shows LBCL-IRF4 have a distinct molecular mutations in IRF4 [8- 10]. Although tumor cells are characterized by high heterogeneous nucleus, and the proliferation index is usually high, but the prognosis is good. We must familiarity with its clinicopathological features, and further studies are needed to define the potential functional effect of these mutations, for what is helpful for diagnosis and differential diagnosis to avoid misdiagnosis and causing overtreatment. GATA3 is critical in controlling lymphoid cell differentiation, but not expressed in normal B cells. Resent study points to new roles of GATA3 in global cellular reprogramming and pathogenesis of B cell malignancies [11]. However, LBCL-IRF4 with GATA3 positive have never been reported.
A 2-year-old patient without underlying disease and any relevant medical history , presented with snoring for several days. Physical examination showed the left tonsillar hypertrophies, and tonsillectomy was performed without any hemorrhagic or infectious complication.
The specimen was fixed with 10% neutral formalin solution, conventional dehydration, transparency, paraffin embedding, sliced thickness 4 μm, and the dyeing was carried out by using the HE method. In addition, the thin sections were resected 2 μm thick and stained with HE to observe the details of tumor cells.
Immunohistochemistry was using EnVision method. The antibodies used are as follows: CD20, CD79a, CD3, CD5, CD45RO, CD21, CD23, MUM-1, GATA3, BCL6, BCL2, CD10, CyclinD1, TDT, CD99, CD38, CD138, Ki-67, purchased from Beijing Zhongshan Jinqiao Biotechnology Co.,Ltd and Fuzhou Maixin Biotechnology Co., Ltd.
Immunohistochemical stains together with Epstein-Barr virus (EBV) was evaluated as part of the diagnostic workup.
FISH analyze was performed formalin-fixed, paraffin-embedded tissue sample according to the local procedure previously described, using three-color probes of mixed IRF4 dual color split probe and IGH 3’ probe.
Male, 2 years old, the tumor is located in the left tonsil.
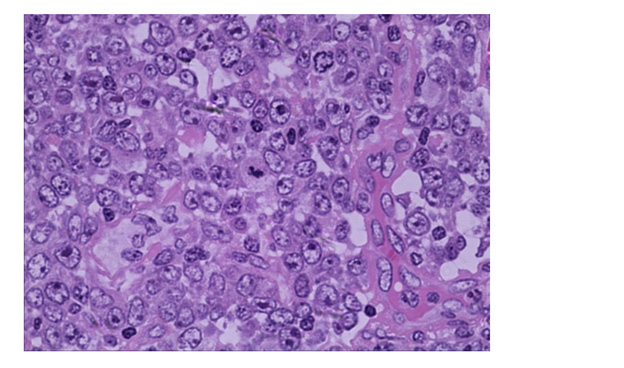
Histologically, the tonsil is replaced by huge, irregular, and confluent follicles. These abnormal follicles are expansive, pushing the surrounding tissues. The mantle zone disappears or becomes narrow and long(Figure 1). Many adjacent atypical germinal centers (GC) enriched in centroblasts but lack polarisation and starry-sky appearance. High magnification showing the neoplastic cells are composed predominantly of medium to large size blasts, have abundant cytoplasm, irregular nuclei, and coarse chromatin vacuole (Figure 2). Few tangible body macrophages can be seen. Some tumor cells have one or more small nucleolus close to the nuclear membrane, basophilic nucleolus can be seen. Sparse and small tumoral necrosis foci are visualized associated with numerous apoptotic cells and mitotic figures.
Figure 1: Hematoxylin & eosin (HE, × 100). Histologically, low magnification showing partial architectural loss of tonsil with irregular follicles growing by pushing and extruding.
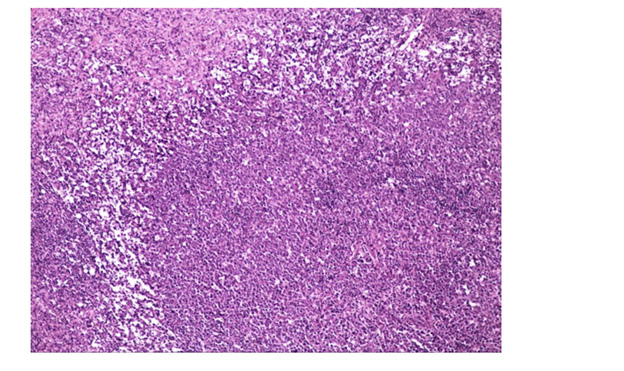
Figure 2: Hematoxylin & eosin (HE, × 400). Morphologically, high magnification showing neoplastic cells have one or more small nucleolus close to the nuclear membrane with centroblastic morphology.
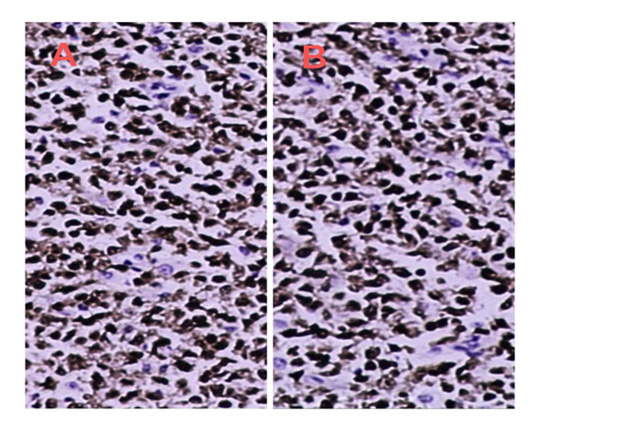
Immunohistochemically, tumor cells positive for mature B cell markers, including CD20 and CD79a. MUM1 and GATA3 is strongly positive (Figure 3), with a Ki-67 index of about 80% (Figure 4). CD21 and CD23 show a few disordered and broken follicular dendritic nets. BCL6 is focally positive, whereas CD3, CD5, CD45RO, CyclinD1, TDT, CD138 are all negative, EBER is also negative.
Figure 3: Evision*200 Immunohistochemical features. A. The lymphoma cells are strong diffuse nuclear positive for MUM1/IRF4. B. The lymphoma cells are strong diffuse nuclear positive for GATA3.
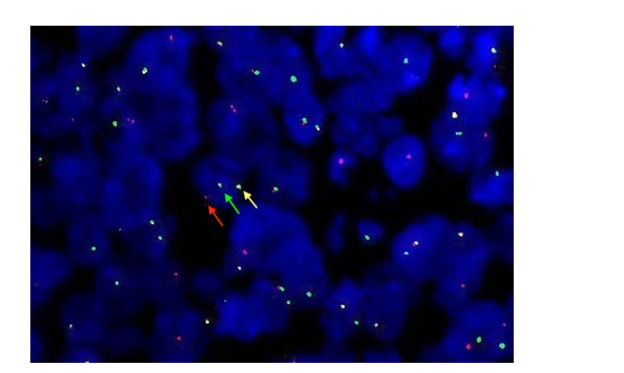
FISH studies use the break-apart IRF4 probe demonstrated a rearrangement on 80% of the cells, with a single yellow signal appeared in the nuclear of tumor cells, and a red and a green signal were isolated (Figure 5).
Figure 5: FISH analysis images use the IRF4 break-apart probe shows a signal constellation of 1 colocalization (yellow arrow) and 1 split signal (red and green arrows) demonstrating the presence of an IRF4-IGH translocation.
Thus, based on these findings, a LBCL-IRF4 characterized by a follicular pattern was diagnosed according to the criteria cited in the 2016 WHO classification. The patient received complete remission after 1 cycle R-CHOP regimen chemotherapy. Follow-up 18 months, the patient is still healthy, but still need more long-term follow-up data.
LBCL-IRF4 described by Salaverria, et al. [4] in 2011, and constituted a novel provisional entity recently included in the 2016 WHO classification of lymphoid tumors [6]. As a special type of mature B-cell tumor, it is accounting for 0.05% of all DLBCL. The predilection sites are head and neck or abdominal regions, genetically characterized by IRF4 translocation, and a favorable outcome after chemotherapy [4,12,13]. Immunohistochemical profile present the mature B-cell phenotype including CD20, CD79a and PAX5, and the most characteristic is the cells strong express IRF4/MUM1. The proliferation index is usually very high, and the expression pattern of Ki-67 shows the neoplastic follicles lack the polarity of normal lymphoid follicles or reactive proliferating follicles.
We present a case of LBCL-IRF4. The clinical, histological, and genetic features reported were consistent with the description proposed in the 2016 WHO classification. Systematic diagnostic approach and focused ancillary studies have led to the correct diagnosis while excluding other pathologies from the differential diagnoses. However, the rare case with tumor cells expressing GATA3. And what role the GATA3 plays in such tumors is unclear and need more cases to be explored.
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
The authors declare that they have no conflict of interest.
Not applicable.
All authors declare no conflict of interest.
None.
Minya Lu performed immunohistochemical analyses, and wrote the manuscript; Lisong Teng provided clinical data; Zhe Wang, Xiaodong Teng and Zhaoming Wang contributed to design the research and support gene expression analyses.


