Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Vladimir N Melnikov*, Valentina V Gultyaeva, Margarita I Zinchenko, Tamara G Komlyagina, Irina V Karmakulova, Dmitriy Yu Uryumtsev and Sergey G Krivoschekov
Received: September 14, 2022; Published: September 23, 2022
*Corresponding author: Vladimir N Melnikov, Scientific Research Institute of Neurosciences and Medicine. 630117. Novosibirsk P.O.Box 237, Russia
DOI: 10.26717/BJSTR.2022.46.007342
Aim: to evaluate cardiovascular responses to acute cycling intervention and hand grip with special reference to left ventricular (LV) systolic and diastolic functions, arterial stiffness and central hemodynamics assessed by aortic Pulse Wave Analysis (ShygmoCor).
Material and Methods: Nine normotensive apparently healthy physically active young men (age 16–32 years) performed a 5-min hand-grip isometric exercise at 20 % of the predetermined maximum grip. Twenty min after the hand grip the subjects underwent 10-min bout of ergometer exercise at smoothly increasing workload with the slope of 10–25 W/min up to the heart rate of 170 beat/min. A paired sample Wilcoxon test was used to assess the differences between baseline and post exercise parameters.
Results: Both exercises increased systolic brachial and central blood pressures whereas diastolic pressures was increased only after the hand grip. The aortic augmentation index increased and time to reflection wave decreased in both cases indicating the stiffening effect of exercises on peripheral arteries. The rate of LV contraction assessed by the increment of aortic pressure per msec tended to elevate after the both kind of exercise (p<0.1). Heart rate and ejection duration increased only after ergometer load. The left ventricular early relaxation time (LVRT) changed from 21.5% of period at rest to 26.1% after the cycling intervention (p<0.001) whereas the relative diastole duration and Buckberg subendocardial viability ratio (SEVR) decreased from 68.2 % to 60.6 % (p<0.001) and from 169 % to 120 % (p<0.001), respectively.
Conclusion: Both types of acute exercise exert hypertensive and stiffening effects determined through peripheral and central systolic pressures and augmentation index. The cycling exercise, but not hand grip, extends the LVRT via the shortening diastole and thus decreasing SEVR. The differences in the effects observed between two exercises are rather qualitative than quantitative.
Keywords: Exercise; Left Ventricular; Contraction; Relaxation; Pulse Wave Analysis
In the most heart pathologies, myocardial relaxation is impaired to a greater extent than contraction. Long left ventricular (LV) relaxation is independently associated with heart failure [Marwick, et al. [1]] and other cardiac pathologies [Gasiorek, et al. [2]]. When added to standard risk factors, slow relaxation improves the prediction of CV pathological events [Kapelko, [3]]. However, it is unclear what are mechanisms of effects of various internal and environmental stressors on relaxation rate. Hypoxia has been found to be among external stressors that slow the LV relaxation [Serizawa, et al. [4]; Melnikov, et al. [5]]. However, Magnani with co-authors have found no impact of mild exercise bout in acute normobaric hypoxia on systolic and diastolic functions [6]. Ryu et al. [7] examined the relationship between everyday physical activity level and prevalence of impaired LV relaxation in large Korean population and found an inverse association by multivariation analysis. Acute exercise is known to increase AIx and decrease SEVR [Doonan, et al. [8]]. Tanaka and co-authors [9] having investigated the influence of isometric and isotonic loads on central and peripheral hemodynamics in men hypothesized that hand-grip exercise is more informative than cycling for evaluating cardiovascular risk. The objective of the present study was to evaluate exercise induced alterations in LV systolic and diastolic functions, central hemodynamics and arterial stiffness and to compare hemodynamic effects of isometric handgrip and ergometer exercises.
Table 1: Characteristics of the participants.
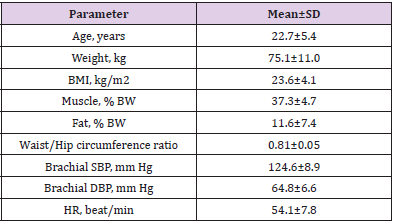
Note: Bmi, Body Mass Index; Bw, Body Weight; Sbp, Systolic Blood Pressure; Dbp, Diastolic Blood Pressure; Hr, Heart Rate
Nine young men aged 16–32 years participated in the study. Table 1 presents the descriptive characteristics of the study subjects. The data characterizes them as normotensive nonobese individuals with normal body fat distribution. All were physically active participating in various regular fitness programs or competitions as unprofessional sportsmen. The Local Ethics Committee of the Institute of Neurosciences and Medicine approved the study protocol. All subjects gave written informed consent to participate in the study.
After 10-min rest in a supine position, the subjects performed a 5-min hand-grip isometric exercise at 20 % of the predetermined maximum grip. Twenty minutes after the hand grip, the subjects underwent 10-min bout of ergometer exercise at smoothly increasing workload with the slope of 15 W/min up to the heart rate of 170 beat/min. Participants pedaled an electronically braked bicycle ergometer under a 12-lead electrocardiogram monitoring. Peripheral blood pressure was measured and central blood pressure was estimated before and 10 min after the exercise in supine position.
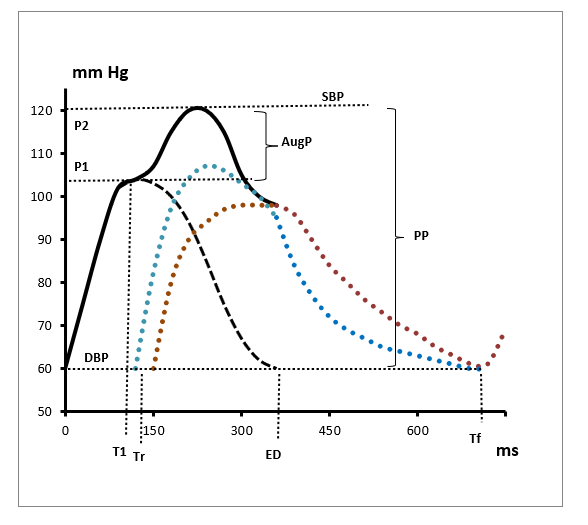
Anthropometric parameters were assessed by the multifrequency tetrapolar bioelectrical impedance analysis device (InBody 370, Korea). The assessment of central hemodynamics and arterial distensibility was performed noninvasively using the SphygmoCor Pulse Wave Analysis (PWA) Px system and SCORPx Version 8.0 software, Type CF (AtCor Medical, Australia) with subjects in a supine position. After recording the blood pressure in the left brachial artery using an automated oscillometric method (Omron M2 Basic, Omron HealthCare Co., Japan), the right radial artery was palpated to identify the point of a suitable pulse. The applanation tonometer was positioned over the artery and the pulse was continuously recorded. For PWA, an average radial pressure waveform was generated from 10 sec of sequential radial pressure waveforms. Using a previously validated generalized transfer function, the system software calculated an averaged radial artery waveform (calibrated with brachial systolic and diastolic pressure) and derived a corresponding aortic pressure waveform, as well as the augmentation pressure and augmentation index adjusted to a heart rate of 75 beat/min. Figure 1 shows the aortic pulse pressure profile. Here we consider the LV systolic function, dividing systole into two phases:
1) Contraction and
2) Early relaxation.
The point T1 indicates the maximum of anterograde wave of aortic pressure that is generated by contracting LV and starts after the opening of aortic valve. This part of the wave corresponds to its ascending shoulder and to rapid ejection. The P1-to-T1 ratio characterizes the intensity of LV contraction or contraction rate (LVCR, mmHg/ms). The subsequent descending branch of the wave, continuing up to the closing aortic valve (ED–T1), reflects the reduced late ejection and the beginning relaxation of LV. The respective LV early relaxation time was calculated according to the following equation: LVRT,% = (ED–T1) / Tf × 100. The augmentation pressure is determined as a rise in pressure mainly due to the reflective wave. In young individuals and persons having compliant arteries, it is common to see no augmentation or a negative one if P2≤P1. Time to reflection, Tr, reflects the timing at the onset of the backward wave coming from peripheral arteries to ascending aorta. It correlates with pulse wave velocity [Wilkinson, et al. [10]] and is considered a proxy or surrogate index of the arterial compliance: the reflected pressure wave arrives earlier in the aortic root from rigid peripheral arteries compared to compliant ones [Vedam, et al. [11]] Buckberg’s subendocardial viability ratio as the diastolic-tosystolic time-power integrals ratio is calculated using the formula
SEVR, % = ∫_ED^Tf▒〖P(t)dt /∫_o^ED▒〖P(t)dt ×100〗〗
and characterizes the myocardial workload and perfusion, i.e. the balance between the energy supply (the first term) and demand.
Note: SBP, DBP, systolic and diastolic blood pressure; AugP, augmentation pressure; PP, pulse pressure; P1, amplitude of antegrade wave; P2, resulted pressure amplitude as a sum of anterograde and return waves; T1, time of maximum anterograde wave; Tr, time of the beginning of retrograde wave; T2, time of second peak pressure; ED, ejection duration; Tf, cardiac cycle duration. Blue line corresponds to retrograde wave from stiff arteries, brown line corresponds to complient arteries.
Figure 1: Aortic pressure profile as a result of Pulse Wave Analysis SphygmoCor.
All statistical analyses were performed using SPSS 19 (Chicago, Il., USA). All numerical data are expressed as Mean±SD. The p-value less or equal to 0.05 calculated by two-sided Wilcoxon test was considered evidence of statistical significance. However, since the work is a pilot study due to small number of subjects we present the p-value ≤ 0.1 to indicate a trend in a difference between baseline and post-exercise parameters.
As Table 2 shows, both exercises substantially increased peripheral and central systolic blood pressures and tended to increase the aortic pulse pressure and LV contraction rate. These results are obvious in view of generally recognized data on the cardiac contractile activation under the physical load. Handgrip load, but not the ergometer exercise, increased the diastolic pressure and did not have any remarkable effect on heart rate. The latter finding allows suggesting that the hypertensive effect of this kind of exercise is due to increase in peripheral vascular resistance but not to positive chronotropic cardiac response. The decreased Tr and the tendency to AP and AIx elevation, proving the arterial stiffening, support this proposition. Certainly, this weak and prompt stiffening cannot be due to alteration in the structure of arteries because of too short duration of the stimuli. In contrast to the grip load, the ergometer exercise, increasing the energy demand and expenditure, markedly increase the HR and does not influence diastolic pressure. The enhanced percent ejection duration is evidently associated with decreased SEVR, this result is in line with the finding of [Doonan, et al. [8]]. Ergometer load usually increases cardiac output and thus the amplitude of forward traveling wave. This in turn is followed by the enhanced extension of peripheral arteries and thus increases the amplitude of backward wave. That is why the exercise increases the augmentation index in more extent than the grip load.
Table 2: Cardiac, hemodynamic and arterial responses to hand-grip and ergometer exercises.
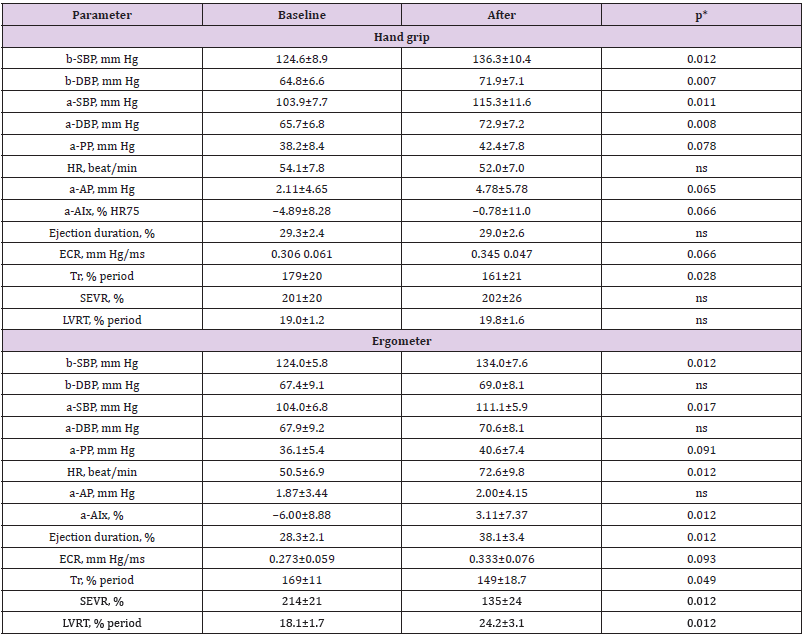
Note: b-, brachial; a-, aortic; SBP, DBP, systolic, diastolic blood pressure; PP, pulse pressure; HR, heart rate; AP, augmentation pressure; AIx, augmentation index; SEVR, subendocardial viability ratio; Tr, time to reflection; ECR, early (left ventricular) contracion rate; LVRT, left ventricular relaxation time. *Wilcoxon test
The important finding of the present study is that ergometer exercise increases the late ejection time and thus slows the early LV relaxation. LV diastolic dysfunction is explored mainly in cardiovascular patients as the predictor of the chronic heart failure. Meanwhile, the slow myocardial relaxation appears to be the common phenomenon of cardiac response to several stimuli. Myocardium, in contrast to skeletal and smooth muscles, is the only muscle in an organism whose contraction-relaxation cycle is forcedly very short. The prolongation of contraction during exercise, associated with the shortening period and reduced relaxation phase, leads to high threat of heart failure. One might speculate that the increasing deficiency of LV relaxation appears to be the cause of cardiac death during intensive exercise of sportsmen having such characteristic deficiency. It is likely that this property is a contraindication to sport. The questions to be answered in future experiments could be as follows: does the exercise-induced extent of LV relaxation slow depend on
(i) The load intensity and hence the value of working cell hypoxia,
(ii) The value of ‘oxygen debt’ accumulated during the exercise, and
(iii) Whether or not the load intensity exceeds the anaerobic threshold?
Not applicable.


