Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Lim Shu Ching and Nabila Perveen and NH Khan*
Received: September 13, 2022; Published: September 23, 2022
*Corresponding author: NH Khan, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, AIMST University, Malaysia
DOI: 10.26717/BJSTR.2022.46.007340
Hyperlipidemia, also known as dyslipidemia and is a medical term for abnormally elevated level of any form of lipids in the blood. The three main types of lipids in human body are phospholipid, triglyceride and cholesterol. Lipids are significant as they assist in essential process such as hormones production, energy storage, regulating and signalling, insulating and protecting, as well as vitamin absorption. However, an excess amount of blood lipids can bring a bad outcome and problems. According to Malaysian Clinical Practice Guidelines (CPGs). Dyslipidemia refers to the following lipid levels in which total cholesterol (TC) level more than 5.2 mmol/L, high-density lipoprotein (HDL) cholesterol less than 1.0 mmol/L in males and less than 1.2 mmol/L in females, triglycerides (TG) more than 1.7 mmol/L. Lastly, low-densitylipoprotein (LDL) cholesterol is depended on the patient’s cardiovascular risk. Cholesterol levels may sometimes be affected by certain medications. For instance, diuretics, birth control pills, corticosteroid and anti-retrovirals used for HIV treatment. Certain health conditions can also contribute to high cholesterol levels, including kidney disease, liver disease, diabetes, and underactive thyroid [1]. Hyperlipidemia can be further subdivided into two classification which is primary (familial) or secondary (acquired) hyperlipidemia. Familial hyperlipidemia can be further sub-divided into five types according to Fredrickson classification as shown in Table 1 which is based upon the pattern of lipoproteins on electrophoresis or ultracentrifugation. It was later adopted by the World Health Organization (WHO).
Hyperlipidemia is a strong risk factor for cardiovascular disease (CVD). CVD has been the leading cause of mortality in both Malaysian men and women for more than a decade [2,3]. CVD includes coronary heart disease (CHD), cerebrovascular disease (strokes) and peripheral arterial disease (PAD). Dyslipidemia remains a significant problem in Malaysia, with the National Health and Morbidity survey in 2015 reporting an estimated 47% of the adult population having hypercholesterolemia. In the main, dyslipidemia is asymptomatic but its associations with serious vascular conditions such as acute myocardial infarction and stroke are well known. [4] There are total of six major groups of lipids modifying drugs that involved in management of dyslipidemia. However, beside modern drug, ever since the dawn of earth, human beings are bank on plants for their survival, including their health benefits. The same trend has been continued even now and approximately 80% of the populations around the globe rely on drugs that are obtained from plant sources. These natural sources were once used for folklore practices, without the knowledge of the active constituent present in them and which was long before the modern medicinal drugs were developed. In traditional Chinese herbal medicine, Morus Rubra (red mulberry) fresh fruits have been used in folk medicine to treat diabetes, hypertension, anemia, and arthritis. Morus rubra is a deciduous tree species from the Moraceae family. It is originated from the eastern part of the United States America and is widely distributed in the moist soils of mesic hardwood forests, floodplains, and other moist sites from south Florida, west to Texas, north to Minnesota and the extreme southern portion of Ontario, Canada, and east to the Mid-Atlantic States. Morus rubra is a deciduous tree that can grow up to 21 m (70 feet). It flower’s from April to May and the seeds are harvested from June to August. The species is dioecious (individual flowers are either male or female, but only one sex is to be found on any one plant so both male and female plants must be grown if seed is required).
The minute flowers are borne in tight catkin clusters. Each fruit develops from an entire flower cluster and is formally known as a multiple. Red mulberry bears on the average 50 multiple fruits per branch and yields about 8.6 fruits/g or 8,600 fruits/kg. In addition, the plant is not self-fertile [5]. Fresh fruits, tree and leaves are shown in Figures 1- 3 accordingly. Recently, the production and consumption of Morus Rubra has increased rapidly because of good taste, nutritional value, and biological activities. Morus Rubra fresh fruits can be used as a warming agent, a remedy for dysentery, and a laxative, odontalgic, anthelmintic, expectorant, hypoglycemic, and emetic [6]. Anthocyanins are the most important constituent of Morus Rubra, which are a group of naturally occurring phenolic compounds that are responsible for the color attribute and biological activities such as antioxidant, antimicrobial, and neuroprotective, anti-inflammatory properties [7]. In recent studies, Morus Rubra also known to be have antihyperlipidemic effect [8]. According to its beneficial properties, Morus Rubra can consider to be developed into nutraceutical which defined as any substance that is a food or part of a food and provides medical or health benefits, including the prevention and treatment of disease. Tables 2 & 3 showed the taxonomical classification and vernacular names of Morus rubra respectively. Tables 4 & 5 shows the organoleptic characteristics and medical uses, respectively.
300g of fresh fruits of Morus Rubra (mulberry) were weighted and spread to allow air drying under room temperature for the duration of 2 days.
Maceration Process of fresh fruits of Morus Rubra was carried out after 2 days of air dried.
a) The required apparatus (Round bottom flask and measuring cylinder) were washed thoroughly 2 times with distilled water and lastly rinsed with ethanol.
b) 250g of fruits of Morus Rubra was chose and weighted from the air-dried fruits and transferred into 1000ml round bottom flask.
c) 550 ml of ethanol was measured using measuring cylinder and transferred into round bottom flask.
d) Fresh fruits of Morus Rubra were completely immensely in the ethanol solution.
e) The mouth of the round bottom flask was covered well with aluminum foil to avoid any evaporation of the ethanol and the active component of the extract.
f) The round bottom flask was swirled for 5 minutes to ensure uniform and complete extraction of mulberry fruits.
g) The round bottom flask was kept at dark, in the cupboard for 9 days and was swirled on daily basis throughout the maceration process.
h) Extra volume of ethanol will be added if ethanol volume was found inadequate to fully maceration the Morus Rubra fruits.
i) After 9 days, the mixture was filtered by using muslin cloth.
j) The round bottom flask was rinsed with ethanol to ensure that all the Morus Rubra fruits was filtered, and no residue left.
k) The filtrate was collected, labelled in a 1000ml beaker and covered up with aluminum foil.
l) The beaker that filled with 400ml extracts was kept in the fridge for further use.
a) Ethanolic maceration extract of fresh fruits of Morus Rubra was underwent evaporation by using rotary evaporator to remove the solvent ethanol present in the extract.
b) The specific collector flask was washed thoroughly 2 times with tap water and rinsed with distilled water.
c) 400ml of extraction in the beaker was transferred into the specific collector flask of the rotary evaporator carefully.
d) The flasks and other equipment were set up accordingly to the procedure of rotary evaporator.
e) The temperature of the heating water bath was set up to 60 °C and the speed of the rotator was set to 100rpm. This evaporation process required approximately 2 hours to be completed.
f) The mass of extraction was measured and collected and laballed in porcelain dish and covered up with aluminum foil for further use.
g) The mass of the weight of the final extract was weight and calculated by follow the equation (a) - (b)= (c)
(a) = Weight of porcelain dish containing Morus Rubra fruit extraction (136.17g)
(b) = Weight of an empty porcelain dish (85g)
(c) = Weight of Morus Rubra fruit extraction (51.17g)
The presence of 25 different phytochemical groups were checked using the ethanolic extract as shown in Table 6.
Table 6: Phytochemical groups identified.
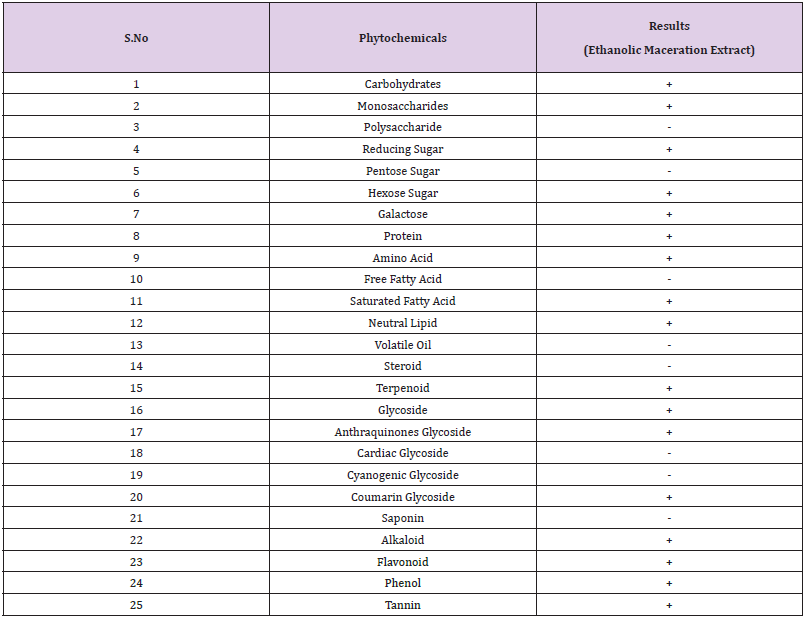
Note: (+) sign indicates the presence of phytochemical, (-) sign indicates the absence of phytochemical.
Preparation and assay of nutrient broths for MIC, MBC, serial dilution procedure for MIC and preparation of nutrient agar plates for MBC test were followed as described already elsewhere by our group research.
a. 36 assay tubes were washed, dried at room temperature and brought for sterilization in hot air oven at 160 ºC for 1 hour.
b. 1.30 g of nutrient broth was weighed using analytical balance.
c. 100 ml of distilled water was measured using measuring cylinder.
d. The weighed quantity of nutrient broth was transferred into a 250 ml of conical flask and dissolved with 100 ml of distilled water.
e. The magnetic stirrer was used to dissolve the nutrient broth completely in the distilled water and little heat was applied at 50-60 ºC through adjusting the hot plate.
f. Total one conical flask with 100 ml of nutrient broth was prepared.
g. 2.0 ml of prepared nutrient broth was transferred to each sterilized assay tube with the help of P1000 micropipette.
h. The mouth of all 36 assays tubes contained the nutrient broth were fitted with cottons and placed neatly in a 500 ml beaker. Then, the mouth of the beaker was covered with aluminium foil.
i. Several 1.0 ml pipette tips were placed in a 500 ml beaker and the mouth of the beaker was covered with the aluminium foil.
j. The 500 ml beakers contained of assay tubes with nutrient broth and 1.0 ml pipette tips together with a pipette tip box consisted of few 0.1 ml pipette tips were subjected to autoclave for 15 minutes at 121 oC for sterilization.
k. The assay tubes with sterilized nutrient broth were allowed to cool down after autoclaved and were ready for MIC test.
M. rubra fruit did not show antibacterial activity against E. coli. and Streptococcus aureus. It is considered not susceptible to gram negative bacterial. The results are laid down in Figures 4-7 respectively where no zone of inhibition was observed. Figure 7 is of MBC on E. coli at 500, 250 and 125 mg/ml concentration. Figure 8 shows the results of MBC on S. aureus at 500, 250 and 125 mg/ml concentration. Figures 9 & 10 shows the results of E. coli and S. aureus where there is no growth.
Figure 5: The fruits were spread on the cardboard and allowed air drying under room temperature under shade.

Figure 9: MBC result of triplicate sets of E. coli and a positive control. There is no observation of bacterial growth.
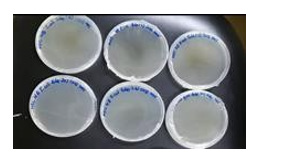
Figure 10: The fruits were spread on the cardboard and allowed air drying under room temperature under shade.
36 healthy, adult male Sprague Dawley rats (180 ± 20 g) were obtained from central animal house, AIMST University, Malaysia. Animal will house in six separates large, spacious polyacrylic cages at ambient room temperature with 12 hours light / 12 hours dark cycle. Animal was allowed to acclimatize for a period of 1 week before utilized in the study. The rats were fed with normal rodent pellets and water ad libitum. The care of animals was carried out according to the guidelines of the AUAEC. The completed animal ethics clearance application form for this study was submitted and approved (AUAEC/FOP/2022/09). Figure 11 gives the information about 36 healthy, adult male Sprague Dawley rats in AIMST central animal house. Figure 12 is showing the feeding of diabetic rat with plant extract by oral gavage.
Figure 11: The fruits were spread on the cardboard and allowed air drying under room temperature under shade.

Figure 12: The fruits were spread on the cardboard and allowed air drying under room temperature under shade.
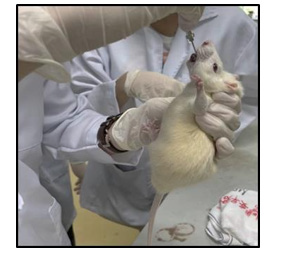
Six groups of adult rats (n =6) were employed in this study. Hyperlipidemia was induced in overnight-fasted rats by intraperitoneal injection of Triton X-100 (100mg/kg) for 3 days [9].
Adult, healthy male Sprague Dawley rats (180 ± 20 g) were used for the experiment. The rats were divided into six different groups of six animals as follows.
Group I – Normal control rats Group II – Hyperlipidemic control
Group III – Hyperlipidemia rats with simvastatin (20mg/kg)
Group IV - Hyperlipidemia rats with Morus Rubra fresh fruits extraction (100mg/kg) Group V - Hyperlipidemia rats with Morus Rubra fresh fruits extraction (200mg/kg) Group VI - Hyperlipidemia rats with Morus Rubra fresh fruits extraction (400mg/kg). After 7 days of study, the blood sample of rats was collected and measured the lipid profile by using refloton analyzer with strips.
The body weight of each rat in each group was recorded on day 1, 3 and 7. The percentage change in body weight was calculated.
A blood sample (0.5 ml) was collected from each experimental animal on the day preceding the commencement of the study and the last day of the study. The samples were drawn from the retroorbital sinus under mild ether anaesthesia [10]. The blood samples were collected in Eppendorf tubes and centrifuged at 10000 RPM for 6 min. The plasma obtained was maintained at 80° C until analysis [11-14].
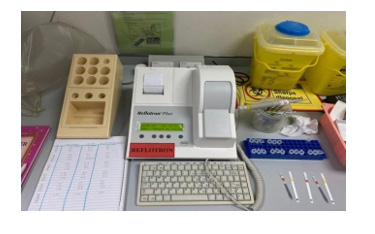
The plasma was used to estimate the plasma lipid profile. The TC, TG and HDL levels were measured using a Reflotron Biochemical Analyzer (Reflotron Plus System, Hoffmann-La Roche, USA) and lipid profile analytical strips. The LDL, VLDL ratio and AD were calculated mathematically. Estimation of plasma lipid profile using a Reflotron biochemical analyzer and lipid profile analytical strip is shown in Figure 13.
Figure 13: Estimation of plasma lipid profile using a Reflotron Biochemical Analyzer and lipid profile analytical strip
All the values are represented as the mean S.E.M. Statistical differences among the groups were determined using One-way repeated measures ANOVA, followed by the Turkey post hoc test. P< 0.05 was considered to be significant.
Effect of ethanolic extract of fresh fruits of Morus Rubra on body weight of Triton X-100 induced hyperlipidemia rats is shown in Tables 7 & 8.
Table 8: Effect of ethanolic extract of fresh fruits of Morus Rubra on body weight of rats.
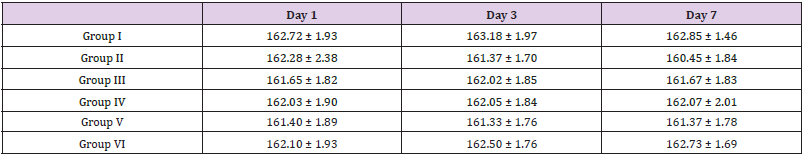
Note: All the values are mean ± SEM (n =6).
Table 9: Lipid profile of rats after triton administration.
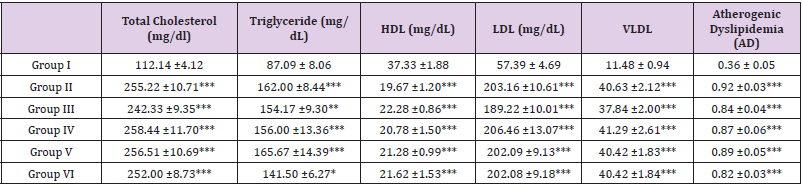
Note: All the values are mean ± SEM (n =6). *P<0.05, **PM<0.01 and ***P<0.001 compare with that of control group (One-way ANOVA followed by Tukey’s post-hoc test)
Table 10: Effect of ethanolic extract of fresh fruits of Morus Rubra on lipid profile of rats at the end of the study.
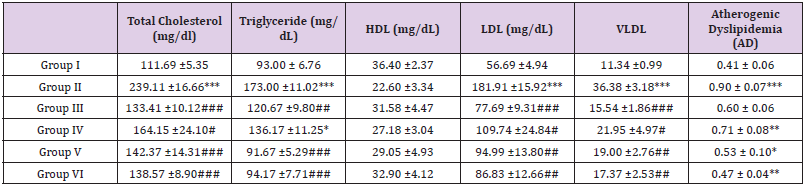
Note: All the values are mean ± SEM (n =6). *P<0.05, **PM<0.01 and ***P<0.001 compare with that of control group; #P<0.05, ##P<0.01 and ###P<0.001 compare with that of hyperlipidaemia group (group II) (One-way ANOVA followed by Tukey’s post-hoc test)
Results of MIC and MBC of Ethanolic Extract: Table 9 showed the result on lipid profile of Triton X-100 induced hyperlipidemia rats after triton administration. There was a significant elevation in total cholesterol, triglyceride, LDL, VLDL and atherogenic dyslipidemia in triton-induced hyperlipidemic rats (Group II, III, IV, V and VI) when compared with normal control (Group I). At this time, an increased level of HDL cholesterol was also observed. Triton showed a significant decrease (P<0.001) in HDL level when compared to the control group (Group I). Table 10 showed the result with ethanolic extract of fresh fruits of Morus Rubra on lipid profile of Triton X-100 induced hyperlipidemia rats at the end of the study. Standard control (Group II) showed significant elevated in TC, TG, LDL, VLDL and AD when compared to normal group (Group I). Group III treated with standard antihyperlipidemic agent simvastatin 10 mg/kg body weight showed significant decreased in TC, TG, LDL, VLDL and AD when compared to hyperlipidemia group (Group II). Group that treated with ethanolic extract of fresh fruits of Morus Rubra (Group IV, V and VI) showed significant serum lipid-lowering effects in Triton- induced hyperlipidemia rats which brought down TC, TG, LDL, VLDL and AD when compared to the hyperlipidemia group (Group II).
Approval from the AIMST University Human and Animals Ethics Committee (AUHAEC/FOP) was obtained prior to the study. This research was conducted in accordance with the recommendations of AUHAEC Guidelines.
The authors are highly thankful to the Faculty of Pharmacy, AIMST University, Bedong, Kedah D.A., Malaysia for funding and providing facilities to carry out this research project.
Thanks to science officers Miss Amalina and Miss Jaya.
Authors declare that there is no conflict of interests.


