Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: September 01, 2022; Published: September 12, 2022
*Corresponding author: Gian Maria Pacifici, Associate Professor of Pharmacology, via Sant’Andrea 32, 56127 Pisa, Italy
DOI: 10.26717/BJSTR.2022.46.007302
Macrolides used in paediatric patients are: erythromycin, clarithromycin, and azithromycin. The oral dose of erythromycin for children is 30 to 50 mg/kg daily divided into 4 portions. Clarithromycin usually is given twice-daily at a dose of 250 for adults with mild-to-moderate infections. In children, the recommended dose of azithromycin oral suspension for treating acute otitis media and pneumonia is 10 mg/kg on the first day (maximum 500 mg) and 5 mg (maximum 20 mg daily) on days 2 through 5. The dosing of erythromycin, clarithromycin, and azithromycin has been extensively described. The efficacy and safety of erythromycin, clarithromycin, and azithromycin have been reported. The pharmacokinetics of erythromycin estolate and ethylsuccinate have been studied in infants and the elimination half-life at the steady state is 6.56 and 2.34 hours, respectively. The pharmacokinetics of clarithromycin have been studied in infants and children, and the elimination half-life is about 4 hours. The pharmacokinetics of azithromycin have been studied in infants and children and the mean elimination half-life is 31.6 hours. The treatment of bacterial infections with erythromycin, clarithromycin, and azithromycin has been reported. Erythromycin, clarithromycin, and azithromycin poorly cross the human placenta and poorly migrate into the breast milk. The aim of this study is to review the dosing, pharmacokinetics, treatment, transfer across the human placenta, and the migration into the breast milk of erythromycin, clarithromycin, and azithromycin.
Keywords: Azithromycin; Breast Milk; Clarithromycin; Dosing; Erythromycin; Pharmacokinetics; Placenta; Treatment
Macrolides used in Paediatric patients are erythromycin, clarithromycin, and azithromycin.
Macrolides are bacteriostatic agents that inhibit protein synthesis by binding reversibly to 50S ribosomal subunits of sensitive microorganisms. Erythromycin does not inhibit peptide bond formation per se but rather inhibits the translocation step wherein a newly synthesized peptidyl tRNA molecule moves from the acceptor site on the ribosome to the peptidyl donor site. Grampositive bacteria accumulate about 100-times more erythromycin than do gram-negative bacteria. Erythromycin has reasonably good activity against streptococci but macrolide-resistant strains of Streptococcus pneumoniae often exists are potentially crossresistant to clindamycin and streptogramins B (quinupristin). Clarithromycin is somewhat less active than erythromycin against Haemophilus influenzae, whereas. Azithromycin and clarithromycin have enhanced activity against Mycobacterium avium-intracellulare, as well as against some protozoa (e.g., Toxoplasma gondii, Cryptosporidium, and Plasmodium species). Clarithromycin has good activity against Mycobacterium leprae. The usual oral dose of erythromycin base ranges from 1 to 2 grams daily, in divided doses, usually given 4 times daily. The 500 mg extended-release formulation of clarithromycin is given as 2 tables once-daily. A single 1-gram dose of azithromycin is recommended for patients with uncomplicated urethral, endocervical, rectal, or epididymal infections. Erythromycin given for 7 days is very effective for treatment of acute infections or for eradicating the diphtheria carrier state. Azithromycin or clarithromycin is recommended for primary prevention of infections due to Mycobacterium aviumintracellulare among patients with HIV infection [1].
The literature search was performed electronically using PubMed as search engine. The following key words were used: erythromycin children, clarithromycin children, and azithromycin children. In addition, the book the Pharmacological Basis of Therapeutics [1] has been consulted.
Administration schedule of erythromycin to newborns and children [2]
Newborns. Give: 12.5 mg/kg 4 times daily.
Oral administration of erythromycin effectively treats susceptible infections in patients with penicillin hypersensitivity (e.g., respiratory-tract infections including Legionella infection), skin, mouth infections, and campylobacter enteritis.
Children aged 1 to 23 months. Give: 125 mg 4 times daily. Increase the dose to 250 mg 4 times-daily, the increased dose may be used in severe infections.
Children aged 2 to 7 years. Give: 250 mg 4 times daily. Increase the dose to 500 mg 4 times-daily, the increased dose may be used in severe infections.
Children aged 8 to 17 years. Give: 250 to 500 mg 4 times daily. Increase the dose to 500 or to 1,000 mg 4 times-daily, the increased dose may be used in severe infections.
Intravenous infusion of erythromycin to treat susceptible infections in patients with penicillin hypersensitivity (e.g., respiratory-tract infections including Legionella infection), skin, mouth infections, and campylobacter enteritis.
Newborns. Give: 10 to 12.5 mg/kg 4 times daily.
Intravenous infusion of erythromycin to treat susceptible infections in patients with penicillin hypersensitivity (e.g., respiratory-tract infections including Legionella infection), skin, mouth infections, and campylobacter enteritis.
Children. Give: 12.5 mg/kg 4 times-daily (maximum per dose = 1 gram).
Oral treatment of erythromycin to treat acute cough (if systemically very unwell or at higher risk of complication).
Children aged 1 to 23 months. Give: 125 mg 4 times-daily for 5 days.
Children aged 2 to 7 years. Give: 250 mg 4 times-daily for 5 days.
Children aged 8 to 17 years. Give: 250 to 500 mg 4 times daily.
Oral administration of erythromycin to treat acute otitis media.
Children aged 1 to 23 months. Give: 125 mg 4 times-daily for 5 to 7 days.
Children aged 2 to 7 years. Give: 250 mg 4 times-daily for 5 to 7 days.
Children aged 8 to 17 years. Give: 250 to 500 mg 4 times-daily for 5 to 7 days.
Oral administration of erythromycin to treat chlamydial ophthalmia.
Newborns. Give: 12.5 mg/kg 4 times daily.
Oral administration of erythromycin to treat chlamydial ophthalmia.
Children aged 1 to 23 months. Give: 125 mg 4 times-daily, increase the dose to 250 mg 4 times-daily in severe infections.
Children aged 2 to 7 years. Give: 250 mg 4 times-daily, increase the dose to 500 mg 4 times-daily in severe infections.
Children aged 8 to 17 years. Give: 250 to 500 mg 4 times-daily, increase the dose to 500 or to 1,000 mg 4 times-daily in severe infections.
Intravenous infusion of erythromycin to treat chlamydial ophthalmia.
Newborns. Give: 10 to 12.5 mg/kg 4 times daily.
Intravenous infusion of erythromycin to treat chlamydial ophthalmia.
Children aged 12 to 17 years. Give: 500 mg 4 times-daily for 14 days.
Oral administration of erythromycin to treat early syphilis.
Children. Give: 12.5 mg/kg for times-daily for 14 days.
Oral administration of erythromycin to treat uncomplicated genital chlamydia and gonococcal urethritis.
Children aged 1 to 23 months. Give: 12.5 mg/kg 4 times daily for 14 days.
Children aged 2 to 11 years. Give: 250 mg twice daily for 14 days.
Children aged 12 to 17 years. Give: 500 mg twice daily for 14 days.
Oral administration of erythromycin to treat pelvic inflammatory disease.
Children aged 1 to 23 months. Give: 12.5 mg/kg 4 times-daily for 14 days.
Children aged 2 to 11 years. Give: 250 mg twice daily for 14 days.
Children aged 12 to 17 years. Give: 500 mg twice daily for 14 days.
Oral administration of erythromycin to prevent and treat pertussis.
Newborns. Give: 12.5 mg/kg 4 times daily.
Oral administration of erythromycin to prevent and treat pertussis.
Children aged 1 to 23 months. Give: 125 mg 4 times daily.
Children aged 2 to 7 years. Give: 250 mg 4 times daily.
Children aged 8 to 17 years. Give: 250 to 500 mg 4 times daily.
Intravenous infusion of erythromycin to prevent and treat pertussis.
Newborns. Give: 10 to 12.5 mg/kg 4 times daily.
Intravenous infusion of erythromycin to prevent and treat pertussis.
Children. Give: 12.5 mg/kg 4 times daily.
Oral administration of erythromycin for prevention of secondary case of diphtheria in non-immune patients.
Children aged 1 to 23 months. Give: 125 mg 4 times-daily for 7 days,
Children aged 2 to 7 years. Give: 250 mg 4 times-daily for 7 days.
Children aged 8 to 17 years. Give: 500 mg 4 times-daily for 7 days.
Oral administration of erythromycin for prevention of secondary case of invasive group A streptococcal infection in penicillin allergic patients.
Children aged 1 to 23 months. Give: 125 mg 4 times-daily for 10 days.
Children aged 2 to 7 years. Give: 250 mg 4 times-daily for 10 days.
Children aged 8 to 17 years. Give: 250 to 500 mg for 10 days.
Oral administration of erythromycin for prevention of pneumococcal infection in asplenia or in patients with sickle-cell disease in patients allergic to penicillin.
Children aged 1 to 23 months. Give: 125 mg twice daily.
Children aged 2 to 7 years. Give: 250 mg twice daily.
Children aged 8 to 17 years. Give: 500 mg twice daily.
Oral administration of erythromycin for prevention of recurrence of rheumatic fever.
Children aged 1 to 23 months. Give: 125 mg twice daily.
Children aged 2 to 17 years. Give: 500 mg twice daily.
Oral treatment of erythromycin to treat acne.
Children aged 1 to 23 months. Give: 250 mg once daily.
Children aged 2 to 17 years. Give: 500 mg twice daily.
Oral administration of erythromycin to treat gastrointestinal stasis.
Newborns. Give: 3 mg/kg 4 times daily.
Oral administration of erythromycin to treat gastrointestinal stasis.
Children. Give: 3 mg/kg 4 times daily.
Intravenous infusion of erythromycin to treat gastrointestinal stasis.
Newborns. Give: 3 mg/kg 4 times daily.
Intravenous infusion of erythromycin to treat gastrointestinal stasis.
Children aged 1 to 11 years. Give: 3 mg/kg 4 times daily.
Oral erythromycin is effective and safe for the treatment of feeding intolerance in very low-birth-weight infants [3]. Oral erythromycin is effective and safe for treatment of feeding intolerance in preterm infants [4]. The prophylaxis of bacterial infection with erythromycin is efficacy and safe in very low-birthweight infants [5]. Low-dose erythromycin is efficacy and safe to treat bacterial infections in premature infants [6]. A 7-day regimen of clarithromycin and a 14-day course of erythromycin are equally effective and safe for treatment of pertussis in children [7].
Patamasucon, et al. [8] studied the pharmacokinetics of erythromycin ethylsuccinate and estolate in 28 infants, aged less than 4 months, and erythromycin estolate or ethylsuccinate was administered at a dose of 10 mg daily. Table 1 summarizes the pharmacokinetic parameters of erythromycin estolate and erythromycin ethylsuccinate. Table 1.Values are the mean + SD, by Patamasucon, et al. [8]. This table shows that erythromycin estolate and erythromycin ethylsuccinate are rapidly absorbed as the absorption half-life is about 1 hour, erythromycin estolate and erythromycin ethylsuccinate are slowly eliminated, and the elimination half-life of erythromycin estolate is longer than that of erythromycin ethylsuccinate.
Table 1: This table shows that erythromycin estolate and erythromycin ethylsuccinate are rapidly absorbed as the absorption half-life is about 1 hour; erythromycin estolate and erythromycin ethylsuccinate are slowly eliminated. Values are the mean + SD, by Patamasucon, et al. [8].
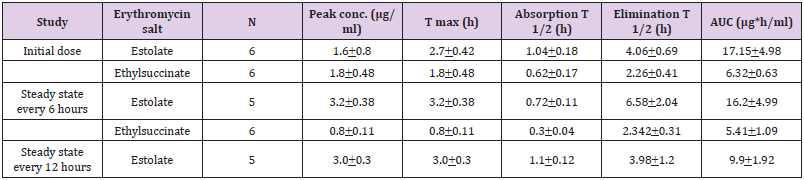
Note: Tmax = time to reach the peak concentration. Absorption T1/2 = absorption half-life. T1/2 = elimination half-life. AUC = area under the concentration-time curve.Treatment of Bacterial Infections with Erythromycin
Early administration of erythromycin significantly reduces the duration of both diarrhoea and faecal excretion in infants and children with acute dysentery associated with Campylobacter Jejuni infection [9]. Erythromycin ethylsuccinate therapy successfully treats Campylobacter enteritis in infants and children [10]. Erythromycin promptly eradicates Campylobacter jejuni from the faeces of infants and children [11]. Erythromycin successfully treats gastroenteritis in infants and children [12].
Intravenous administration of erythromycin at a dose of 600 mg achieves therapeutic concentration in maternal serum whereas erythromycin lacks therapeutic concentration in umbilical vein serum [13]. The transfer of erythromycin across the human placenta was studied in 31 pregnant women at delivery. The concentration of erythromycin in the umbilical cord vein plasma is 3.0% of that in the maternal plasma [14].
Two women were treated with a single dose of 500 mg erythromycin orally and milk concentrations of erythromycin were measured at 2, 4, and 6 hours after the dose and are 1.0, 1.2, and 1.1 μg/ml, respectively. These results indicate that erythromycin poorly migrates into the breast milk [15].
Oral administration of clarithromycin to treat respiratory-tract infections, mild-to-moderate skin, and soft-tissue infections.
Newborns. Give: 7.5 mg/kg twice daily.
Oral administration of clarithromycin to treat respiratory-tract infections, mild-to-moderate skin, and soft-tissue infections.
Children aged 1 month to 11 years, with body weight up to 8 kg. Give: 7.5 mg/kg twice daily.
Children aged 1 month to 11 years, with body weight of 8 to 11 kg. Give: 62.5 mg twice daily.
Children aged 1 month to 11 years, with body weight of 12 to 19 kg. Give: 125 mg twice daily.
Children aged 1 month to 11 years, with body weight of 20 to 29 kg. Give: 187 mg twice daily.
Children aged 1 month to 11 years, with body weight of 30 to 40 kg. Give: 250 mg twice daily.
Children aged 12 to 17 years. Give: 250 mg twice daily for 7 to 14 days. Increase the dose to 500 mg in severe infections (e.g., pneumonia).
Intravenous infusion of clarithromycin to treat respiratorytract infections, mild-to-moderate skin, and soft-tissue infections.
Children aged 1 month to 11 years. Give: 7.5 mg/kg twice daily.
Children aged 12 to 17 years. Give: 500 mg twice daily.
Oral administration of clarithromycin to treats acute exacerbations of bronchiectasis.
Children aged 1 month to 11 years, with body weight up to 8 kg. Give: 7.5 mg/kg for 7 to 14 days.
Children aged 1 month to 11 years, with body weight of 8 to 11 kg. Give: 62.5 mg twice daily for 7 to 14 days.
Children aged 1 month to 11 years, with body weight of 12 to 19 kg. Give: 125 mg twice daily for 7 to 14 days.
Children aged 1 month to 11 years, with body weight of 20 to 29 kg. Give: 187 mg twice daily for 7 to 14 days.
Children aged 1 month to 11 years, with body weight of 30 to 40 kg. Give: 250 mg twice daily for 7 to 14 days.
Children aged 12 to 17 years. Give: 250 to 500 mg twice daily for 7 to 14 days.
Oral administration of clarithromycin to treat acute cough (if systemically very unwell or at higher risk of complications).
Children aged 1 month to 11 years, with body weight up to 8 kg. Give: 7.5 mg/kg for 5 days.
Children aged 1 month to 11 years, with body weight of 8 to 11 kg. Give: 62.5 g/kg twice daily for 5 days.
Children aged 1 month to 11 years, with body weight of 12 to 19 kg. Give: 125 mg twice daily for 5 days.
Children aged 1 month to 11 years, with body weight of 20 to 29 kg. Give: 187 mg twice daily for 5 days.
Children aged 1 month to 11 years, with body weight of 30 to 40 kg. Give: 250 mg twice daily for 5 days.
Children aged 12 to 17 years. Give: 250 to 500 mg twice daily for 5 days.
Oral administration of clarithromycin to treat acute otitis media.
Newborns. Give: 7.5 mg/kg twice daily.
Oral administration of clarithromycin to treat acute otitis media.
Children aged 1 month to 11 years, with body weight up to 8 kg. Give: 7.5 mg/kg for 5 to 7 days.
Children aged 1 month to 11 years, with body weight of 8 to 11 kg. Give: 62.5 mg twice daily for 5 to 7 days.
Children aged 1 month to 11 years, with body weight of 12 to 19 kg. Give: 125 mg twice daily for 5 to 7 days.
Children aged 1 month to 11 years, with body weight of 20 to 29 kg. Give: 187 mg twice daily for 5 to 7 days.
Children aged 1 month to 11 years, with body weight of 30 to 40 kg. Give: 250 mg twice daily for 5 to 7 days.
Children aged 12 to 17 years. Give: 250 to 500 mg twice daily for 5 to 7 days.
Oral administration of clarithromycin to prevent pertussis.
Newborns. Give: 7.5 mg/kg for 7 days.
Oral administration of clarithromycin to prevent pertussis.
Children aged 1 month to 11 years, with body weight up to 8 kg. Give: 7.5 mg/kg for 7 days.
Children aged 1 month to 11 years, with body weight of 8 to 11 kg. Give: 62.5 mg twice daily for 7 days.
Children aged 1 month to 11 years, with body weight of 12 to 19 kg. Give: 125 mg twice daily for 7 days.
Children aged 1 month to 11 years, with body weight of 20 to 29 kg. Give: 187 mg twice daily for 7 days.
Children aged 1 month to 11 years, with body weight of 30 to 40 kg. Give: 250 mg twice daily for 7 days.
Children aged 12 to 17 years. Give: 500 mg twice daily for 7 days.
Oral administration of clarithromycin for Helicobacter pylori eradication in combination with omeprazole, and amoxicillin or metronidazole.
Children aged 1 to 5 years. Give: 7.5 mg/kg twice daily.
Children aged 6 to 11 years. Give: 7.5 mg/kg twice daily.
Children aged 12 to 17 years. Give: 500 mg twice daily.
Oral administration of clarithromycin to treat acute sinusitis.
Children aged 1 month to 11 years, with body weight up to 8 kg. Give: 7.5 mg/kg for 5 days.
Children aged 1 month to 11 years, with body weight of 8 to 11 kg. Give: 62.5 mg twice daily for 5 days.
Children aged 1 month to 11 years, with body weight of 12 to 19 kg. Give: 125 mg twice daily for 5 days.
Children aged 1 month to 11 years, with body weight of 20 to 29 kg. Give: 187 mg twice daily for 5 days.
Children aged 1 month to 11 years, with body weight of 30 to 40 kg. Give: 250 mg twice daily for 5 days.
Children aged 12 to 17 years. Give: 250 mg twice daily for 5 days.
Clarithromycin is a safe and effective agent for treatment of upper respiratory-tract infections in infants and children [17]. Clarithromycin is a safe and effective antimicrobial agent for treatment of acute otitis media in children [18]. Clarithromycin is more effective and safer than amoxicillin/clavulanate for treatment of acute otitis media in children [19]. A 10-days regimen of clarithromycin suspension (7.5 mg/kg twice-daily) is safe and effective as 10-days regimen of ceflacor suspension (20 mg/kg twice-daily) for treatment of acute otitis media in children [20].
Gan et al. [21] studied the pharmacokinetics of clarithromycin in 24 infants and children aged 6 months and 10 years (mean, 3.3 years) and weighing 6.7 to 14.7 kg (mean, 14.7). Subjects of group 1 and 2 received a clarithromycin suspension at 7.5 mg/kg once daily, group 1 under fasting condition and group 2 under nonfasting states. Group 3 subjects were in the multiple-dose study and received clarithromycin at a dose of 7.5 mg/kg twice-daily. Table 2 provides the demographic characteristics of the subjects included in the study and Table 3 summarizes the pharmacokinetic parameters of clarithromycin. Table 2. Demographic characteristics of the subjects included in the study. Values are the mean, by Gan, et al. [21]. This table shows that clarithromycin is slowly absorbed following oral administration as the time to reach the peak concentration is about 3 hours and clarithromycin is slowly eliminated as the elimination half-life is about 4 hours.
Table 2: Demographic characteristics of the subjects included in the study. Values are the mean, by Gan, et al. [21].
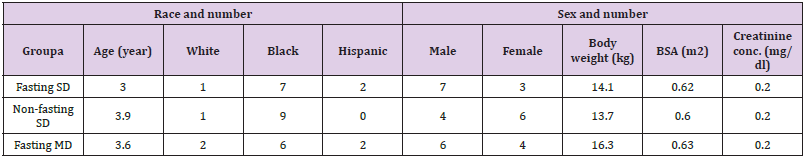
Note: aFor each group N = 10. SD = single dose. MD = multiple doses.
Table 3: Pharmacokinetic parameters of clarithromycin which have been obtained in 24 infants and children. Values are the mean +SD, by Gan, et al. [21].
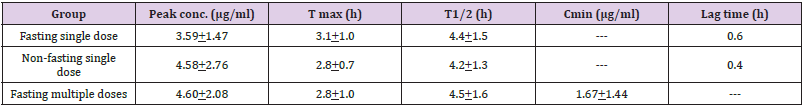
Note: T max = time to reach the peak concertation. T1/2 = elimination half-life. C min = minimum concentration.
Clarithromycin treatment in very-low-birth-weight preterm infants improves enteral feeding [22]. Clarithromycin given twicedaily is safe and effective as given thrice-daily in treatment of acute otitis media in infants and children [23]. Clarithromycin is safe and well-tolerated for the treatment with common paediatric infections even when administered at higher than recommended dose [24]. Clarithromycin therapy reduces mucosal tumour necrosis factor α, interleukin-1β, and interleukin-10 concentrations in children with an acute exacerbation of recurrent wheezing [25]. Administration of clarithromycin is a suitable treatment for improving lower respiratory infections in infants and children caused by Chlamydia pneumoniae [26].
A total of 34 cord-maternal serum pairs were included in the study. The mean cord to maternal serum clarithromycin concentration percentage is 7.93+0.9%. There is a correlation between cord serum and maternal serum clarithromycin concentration (r = 0.795, P<0.001). The cord to maternal serum clarithromycin concentration percentage significantly increases according to advancing gestation (P<0.001). The mean placental transfer of clarithromycin is approximately 8% and is dependent on gestational age [27].
Twelve mothers received clarithromycin orally at a dose of 250 mg twice daily for puerperal infections. The peak concentration of clarithromycin in the milk is 0.85 μg/ml at 2.2 hours after the dose. The elimination half-life of clarithromycin in the milk is 4.3 hours. These results indicate that clarithromycin poorly migrates into the breast milk [28].
Oral administration of azithromycin to prevent secondary case of invasive group A streptococcal infection in children who are allergic to penicillin.
Children aged 6 months to 11 years. Give: 12 mg/kg one-daily for 5 days.
Children aged 12 to 17 years. Give: 500 mg once daily for 5 days.
Oral administration of azithromycin to treat respiratory-tract infection, otitis media, skin, and soft-tissue infections.
Children aged 6 to 17 years, with body weight of 15 to 25 kg. Give: 200 mg once daily for 3 days.
Children aged 6 months to 17 years, with body weight of 26 to 35 kg. Give: 300 mg once daily for 3 days.
Children aged 6 months to 17 years, with body weight of 36 to 45 kg. Give: 400 mg once daily for 3 days.
Children aged 6 months to 17 years, with body weight of 46 kg and above. Give: 500 mg/kg once daily for 3 days.
Oral treatment of azithromycin to treat infections in children with cystic fibrosis.
Children aged 6 to 17 years, with body weight of 25 to 40 kg. Give: 250 mg thrice weekly.
Children aged 6 to 17 years, with body weight of 41 kg and above. Give: 500 mg thrice weekly.
Oral administration of azithromycin to treat uncomplicated genital chlamydia infections and gonococcal urethritis.
Children aged 12 to 17 years. Give: 1 gram for 1 dose.
Oral administration of azithromycin to treat Lyme disease, erythema migrans, and focal symptoms.
Children aged 1 month to 11 years, with body weight up to 51 kg. Give: 10 mg/kg daily for 17 days.
Children aged 1 month to 11 years, with body weight of 51 kg and above. Give: 500 mg daily for 17 years.
Children aged 12 to 17 years. Give: 500 mg daily for 17 days.
Oral administration of azithromycin to treat mild-to-moderate typhoid infection due to multiple antibacterial resistant organisms.
Children aged 6 months to 17 years. Give: 10 mg/kg once daily for 7 days.
Azithromycin given to infants, aged 1 to 5 months, is efficacy and safe in treating community-acquired pneumonia [30]. Azithromycin in the treatment of children with respiratory tract infections if highly effective and safe and causes few adverse effects [31].
Nahata, et al. [32] studied the pharmacokinetics of azithromycin in 13 infants and children with acute otitis media, aged 6 months to 5 years (mean, 2.2+1.8 years), and azithromycin was administered orally at a dose of 10 mg/kg once daily for 5 days. Table 4 summarizes the pharmacokinetic parameters of azithromycin.
Table 4: Pharmacokinetic parameters of azithromycin which have been obtained in 13 infants and children, azithromycin was administered orally at a dose of 10 mg/kg once daily for 5 days. Values are the minimum, maximum, mean, and +SD, by Nahata, et al. [32].
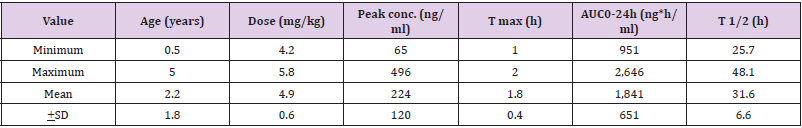
Note: T max = time to reach the peak concentration. AUC = area under the concentration-time curve. T1/2 = elimination half-life. This table shows that azithromycin is rapidly absorbed following oral dosing as the time to reach the peak concentration ranges from 1 to 2 hours and azithromycin is lowly eliminated as the mean elimination half-life is 31.6 hours. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by a wide variation in subject’s age and disease.
Table 4. Pharmacokinetic parameters of azithromycin which have been obtained in 13 infants and children, azithromycin was administered orally at a dose of 10 mg/kg once daily for 5 days. Values are the minimum, maximum, mean, and +SD, by Nahata et al. [32]. This table shows that azithromycin is rapidly absorbed following oral dosing as the time to reach the peak concentration ranges from 1 to 2 hours and azithromycin is lowly eliminated as the mean elimination half-life is 31.6 hours. In addition, there is a remarkable interindividual variability in the pharmacokinetic parameters and this variability is accounted by a wide variation in subject’s age and disease.
Adverse effects following a single oral dose of azithromycin in preschool children are rare and mild and azithromycin is efficacy and safe in this population [33]. Compared to treatment with azithromycin alone, pidotimod combined with azithromycin significantly reduces the expression levels of interlukine-10 and G-CSF in serum of children with mycoplasma pneumonia, improves the curative effect, and reduces the occurrence of adverse-effects [34]. A 4 to 6-month trial of azithromycin is justified in children with cystic fibrosis who do not respond to conventional treatment [35]. Among children with histories of recurrent severe lower respiratory-tract infections the use of azithromycin is more effective than placebo in reducing the likelihood of severe respiratorytract infections [36]. At the end of therapy, clinical cure of acute respiratory-tract infections is achieved in 73 out of 77 children (95%) who received azithromycin, and in 60/72 of children (83%) who received erythromycin thus azithromycin is more effective than erythromycin in treatment of acute respiratory-tract infections in children [37]. A 3-day course of azithromycin is effective and well-tolerated as a 10-day course of clarithromycin in children with acute upper respiratory-tract infections [38]. Azithromycin is an effective, safe and well-tolerated drug in the treatment of children with lower respiratory-tract infections [39].
The transfer of azithromycin across the human placenta was studied in 31 pregnant women at delivery and the concentration of azithromycin in umbilical cord vein plasma is 2.6% of the maternal plasma concentration [14].
A woman at 1 day postpartum received 1 gram of azithromycin orally and forty-eight hours later her milk azithromycin is 0.64 μg/ ml. A woman received azithromycin orally at a dose of 500 mg for 5 days and the milk concentration of azithromycin is 1.30 μg/ml after the first dose and 2.8 μg/ml after the third dose [40]. Thirty women received azithromycin intravenously at a dose of 500 mg daily and the milk concentration of azithromycin is 1.7 μg/ml at 30 hours after the dose [41]. These results indicate that azithromycin poorly migrates into the breast milk.
Macrolides used in Paediatric patients are erythromycin, clarithromycin, and azithromycin. The oral dose of erythromycin for children is 30 to 50 mg/kg daily divided into 4 portions. Clarithromycin is usually given twice-daily at a dose of 250 mg for adults with mid-to-moderate infections. In children, the recommended dose of azithromycin oral suspension for acute otitis media and pneumonia is 10 mg/kg on the first day (maximum 500 mg) and 5 mg (maximum 20 mg daily) of days 2 through 5. Macrolides are suitable drugs for the treatment of acute otitis media, community-acquired pneumonia, respiratory-tract infections, erysipelas, and cellulitis [1]. The dosing of erythromycin, clarithromycin, and azithromycin has been extensively described. The efficacy and safety of erythromycin [3-7], clarithromycin [17- 20], and azithromycin [30-31] have been reported. The elimination half-life of erythromycin estolate and ethylsuccinate has been determined in infants and the elimination half-life of erythromycin estolate and ethylsuccinate are 6.58 and 2.34 hours, respectively, at steady state [8]. The elimination half-life of clarithromycin is about 4 hours in infants and children [21]. The half-life of azithromycin is about 32 hours in infants and children [32]. The treatment of bacterial infections has been studied with erythromycin [9-12], clarithromycin [22-26], and azithromycin [33-39] and these drugs treat the infections caused by different bacteria. Erythromycin [13,14], clarithromycin [27], and azithromycin [14] are poorly transferred across the human placenta and erythromycin [15], clarithromycin [28], and azithromycin [41] poorly migrate into the breast milk.
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals.
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.


