Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Guggenbichler S1 and Guggenbichler JP2,3*
Received: August 01, 2022; Published: August 16, 2022
*Corresponding author: Guggenbichler JP, Em. Prof. Department of Pediatrics, University Erlangen, Germany, CEO: AmiSTec GMbH und Co KG, Kössen, Austria
DOI: 10.26717/BJSTR.2022.45.007240
Clinical environments provide an ideal reservoir for the growth, proliferation, and transmission of pathogenic organisms. Surfaces in hospitals e.g., hospital furniture, ECG lead wires and other cables, push buttons of infusion pumps, control knobs of ventilation machines, textiles as well as implantable biomaterials like central venous catheters, urologic catheters, endotracheal tubes are contaminated increasingly frequent with multiresistant microorganisms. These microorganisms are distributed by the hands of the nursing personnel throughout the hospital with serious, life-threatening consequences. 1.8 million patients suffer from a nosocomial infection per year in Europe; approximately 180,000 deaths are attributed to these infections. The Centers for Disease Control (CDC) estimates that 2 million U.S. patients per year acquire a hospital-related infection. These infections caused 90,000 deaths each year and cost an average of $47,000 per patient to treat. The added cost to hospitals is $4.8 billion anսally for extended care treatment. Microorganisms show an increasing rate of resistance against the majority of antibiotics including carbapenems as last available antibiotic. 700,000 deaths have been rеρorted worldwide, 30 000 deaths alone in Europe during the last year due to infections where no effective antimicrobial substance was available. The use of disinfectants is ostensibly intended to remove/kill pathogens on surfaces. However, studies have shown that more than one-half the time, surfaces are not adequately cleaned or are re-contaminated within minutes. Much emphasis has been put therefore on hand disinfection. However, there are also rеρorts of the emergence of alcohol tolerant/insensitive microorganisms e.g., vancomycin resistant enterococci. This phenomenon has the potential to undermine the effectiveness of alcohol based disinfectant standard precautions. The reason for this dramatic development of resistant microorganisms is still in debate. The indiscriminate use of antibiotics for prevention of a bacterial superinfections e.g. sinusitis, otitis media after a viral infections is frequently incriminated, however this seems to have little impact on the occurrence of multi-resistant hospital pathogens in general. However individual patients are seriously affected by the selection of multi-resistant pathogens. In contrast there is increasing evidence that the widespread use of disinfectants is responsible: disinfectants - analogous to antibiotics - must be incorporated into the metabolism of microorganisms. This is inevitably associated with induction of resistance by transfer of resistance plasmids e.g. induction of efflux pumps. 7880 рսblications (PUBMed Jan 2022) are available in the international literature which document the resistance of microorganisms against disinfectants; 649 рublications describe the cross resistance with antibiotics, at the same time there are 10 819 reports on the toxicity of disinfectants.
The worldwide increase of multi-resistant microorganisms is responsible for millions of deaths per year. 4.95 million deaths according to the recent study were linked to an antibioticresistant bacterial infection. 1.27 million people died directly from infection with a resistant bacterium - so without resistance, these deaths would have been preventable [1]. In the “Report on Antibiotic resistance” bacterial resistance was seen as one of the most common cause of death worldwide and requires immediate, innovative and ambitious action [2-4]. Much emphasis is therefore based on the prevention of nosocomial infections with multi resistant microorganisms. The crucial initial step however is the investigation of the reasons for development of multi drug-resistant microorganisms (MDR). John E. Walker, a Nobel price laureate, reports on behalf of the WHO reasons on this development in 2015: [5].
Viral infections of the upper respiratory tract are frequently observed in children and predispose patients for bacterial superinfections e.g. sinusitis, otitis media or bronchitis, rarely pneumonia or a bloodstream infections where the administration of antibiotics is mandatory [6,7]. Antibiotics have to be administered only once a bacterial superinfection has been documented. This requires frequent clinical controls of the patient. Prophylactic antibiotics are not helpful for the prevention of these bacterial superinfections; in essence they also complicate the treatment of these infections as already resistant microorganisms may have been selected in the oral/nasal flora within a few days. Prophylaxis of bacterial superinfections is feasible with an approach other than antibiotics. Effective alternatives are e.g. anti-inflammatory properties based on herbal extracts (thyme, gentian, primula) which open clogged paranasal sinus openings as well as the patency of the Eustachian tube and improve the mucociliary clearance [8]. These herbal extracts decrease the arachidonic acid metabolism, the precursor of proinflammatory cytokines [9]. This has been documented beyond reasonable doubt by prospective, randomised double blinded studies Table 1.
The use of antibiotics in animal health seems to be an important reason for the development of resistant microorganisms. Antibioticresistant bacteria associated with animals may be pathogenic to humans, easily transmitted to humans via food chains, and widely disseminated in the environment via animal wastes [10]. There are however additional facets to be considered. Antibiotics are generally not found in meat except in broilers as non-absorbable antibiotics e.g. colistin are used [11,12]. The problem is the selection of resistant microorganisms in the excretions which are usually distributed into the environment as fertilisers. Vegetables harvested on these fields are frequently contaminated. The majority of remaining microorganisms on vegetables is eradicated by the hydrochloric acid in the stomach. Some microorganisms escape and are found in the flora of the large intestine in low concentrations. There these microorganisms are under control of a stabile faecal flora. However, if a broad-spectrum antibiotic is administered to the patient, the sensitive flora is eliminated, and the resistant microorganisms prevail. There is also a solution for elimination of prophylactic antibiotics as growth enhancers [13]. The pathogenic property of microorganisms responsible for nosocomial infections is the formation of toxins of the pathogen but also - equally important - adherence on epithelial cells. The blockage of adherence of microorganisms on epithelial cells by receptor analogue carbohydrates i.e. acid galacturonides is an important treatment option and can prevent the use of antibiotics. Animal studies demonstrate superior outcomes of this option compared to antibiotics without induction of resistance.
The lack of attention of pharmacodynamic and pharmacokinetic properties of new antibiotics by regulatory authorities is a severe shortcoming. Broad spectrum antibiotics with incomplete bioavailability (Cefixim) or prodrugs with predominant biliary elimination (Cefuroxime proxetil) select multi resistant microorganisms in the faecal flora just as i.v. Ceftriaxone with a 75% biliary elimination [14]. Azithromycin has an elimination half-life of 68 hours. Subinhibitory concentration in the oral cavity over 3 weeks select invariably high numbers of macrolide resistant microorganisms in contrast with Clarithromycin with a halflife of 2 hours. Macrolide insensitive microorganisms after administration of Azithromycin are also distributed to all classmates in kindergarten [15]. Multi-drug resistant (MDR) bacteria have been reported as contaminating microorganisms of surfaces, commonly used medical equipment and high-contact communal surfaces (e.g., telephones, keyboard, medical charts) in a hospital. Contamination of inanimate surfaces in ICU has been identified in outbreaks and cross-transmission of pathogens among critically ill patients. Inanimate surfaces and equipment (e.g., bedrails, stethoscopes, medical charts, ultrasound machine) are frequently contaminated by bacteria, including MDR isolates. Contamination may occur either by transfer of microorganisms contaminating healthcare workers’ hands or direct patient shedding of microorganisms in the immediate environment of a patient’s bed [16,17]. Inadequate hygiene in hospitals has a proven impact on the development of resistant microorganisms. However strong emphasis, based on alcoholic hand hygiene, has been reported responsible for a massive increase of vancomycin resistant and alcohol insensitive enterococci [18].
The most important reason for this dreadful development is the use of disinfectants. Disinfectants are widely used for elimination of microorganisms from a surface but proved to be a not reliable method any more due to a substantial increase of multi-resistant microorganisms against disinfectants and cross resistance with antibiotics. It has been reported that both Gram-positive and Gramnegative bacteria are able to survive up to months on dry inanimate surfaces, with even longer persistence under humid and lower temperature conditions. Environmental contamination by fungi and viral pathogens including coronavirus has been also described on surfaces in frequented customer areas viable for weeks [19]. Factors that may affect the transfer of microorganisms from one surface to another and cross-contamination rates are type of organisms, source and destination of surfaces, humidity level, and size of inoculum [20,21]. The development of self-sanitizing surfaces with a broad spectrum of activity, fast eradication of microorganisms, long lasting to permanent antimicrobial activity without induction of resistance is the only promising solution for this problem if all the requirements for the prevention of hospital acquired infections are met. The requirements of self-sanitizing surfaces for the prevention of hospital acquired infections in hospitals, public transportation, the food industry are extraordinary high.
Intensive, fast and broad antimicrobial activity, against Grampositive, Gram-negative microorganisms, irrespective of their antibiotic susceptibility, fungi, legionella, moulds, virus documented by the RODAC plate method
Fast eradication of microorganisms i.e. minimum 5 log 10 reduction within 30 minutes.
Activity against a high inoculum size of 109 CFU on an area of 3 cm²
No induction of resistance
Nontoxic, skin and soft tissue compatibility, no allergenicity, sbD (safe by design)
Long lasting/permanent antimicrobial activity water-, acid-, alkaline-, alcohol insoluble, UV light stabile
Cleanable with detergents
Uncomplicated technical processability, heat stabile up to 400°C, non-corrosive
Physical stability, activity irrespective of sweat, grease, blood, pus
Not flammable, smoke reduction
BP authorization by the European commission on biocidal products.
Favourable cost/benefit analysis
To combat multi-drug resistant (MDR) superbugs, a plethora of novel methods are under investigation, while old and momentarily forgotten strategies (nano-compounds, bacteriophages, physical factors) are being revised. There are however several shortcomings of these technologies. Physical factors, such as UV light, high steam temperature have been propagated, especially in indSustries with a high risk of microbial contamination. UV light could kill a wide array of microorganisms including both vegetative and spore forming pathogens by induction of oxygen radicals. However, the activity is often not sufficient for bacterial eradication and has a number of adverse events like skin and eye problems and carcinogenicity [22]. Considerable variabilities in duration and the distance of the light emitting source have been determined. Steam shows an immediate germfree surface – however it does not exhibit a lasting antimicrobial activity. If someone touches the surface after 10 minutes, the surface is contaminated again. The steam technology would have to be reapplied in frequent i.e. in 30 minutes intervals. Various technologies with a potential to curb the dramatic increase of multiresistant microorganisms have been described. Since the early 1900s, bacteriophages are recommended for medical purposes. Several companies and research laboratories pursue a treatment strategy involving phages in infections caused by Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli [23,24]. In some countries (e.g., Russia, Georgia, Poland, USA), bacteria which do not respond to conventional antibiotics are treated by phages. The problem with phages is their specific activity against a certain bacterium. This requires the identification of the microorganisms prior to the application and selection of the suitable phage among thousands of phages. A possible solution might be the use of a phage cocktail containing a multitude of different phages. Phages are not heat resistant and cannot be incorporated into polymers.
There are high expectations of the efficient killing of bacteria using nanostructures and their non-chemical mechanisms such as contact killing, mechanical puncturing, and changes in the local microenvironment via nanoions [25]. Combination with metal oxides, silver, chitosan, gallic acid nanoparticles etc. have also been recommended. Addition of silver is a feasible approach but - as already described - only free silver ions are active – in other words silver has to be released from the hydrophilic bottom layer and incorporated into the bacterial metabolism. The duration of antimicrobial activity is therefore limited to 14 days. Nanoparticles combined with antibacterial agents are also being studied [25]. Antibiotics however are not suitable for providing a permanent endowment of surfaces with antimicrobial properties. The induction of resistance is a great threat. The activity of a technology on the basis of Titanium oxide can only be documented with the JIS 25923 method. The activity is determined in the capillary space between the surface and a foil which prevents the evaporation of oxygen radicals. The determination of antimicrobial activity by the RODAC plate methods shows no efficacy! This method lacks clinical relevance. In situ generated biocides by catalysts (molybdenum oxide, tungsten blue oxide, Zinc molybdate and polyoxometallates) show fast antimicrobial activity against a very broad spectrum of bacterial pathogens including microorganisms embedded in a biofilm, fungi, moulds and several viral pathogens including COVID 19 on surfaces [26-28].
Transition metal oxides can be incorporated into various coatings and polymers, they are water insoluble and have a documented duration of activity of at least 10 years and 10 000 cleanings. Transition metal oxides are not toxic, there is no induction of resistance. The activity and marketability has been documented by external laboratories and the BPR of the EU. In situ generated biocides on surfaces by transition metal oxides meet all the requirements described above. Surfaces decorated with metal oxides eg. Lewis acids such as MoO3, oxygen deficient tungsten blue oxide WO3 and Zinc molybdate have also shown a broad-band and strong antimicrobial activity resulting in a reduction of the number of colony forming units by 6 – 7 log 10 within 1-3 hours. Their mechanism of action is based on the in-situ generation of 4 mechanisms which work in a synergistic mode.
a) H3O+ ions through the reaction with moisture from the air inspired by the body’s own defense mechanism imitating e.g. the acid coating of the skin [29]. The resulting acidified surfaces have a pH of 4.5 and the H3O+ ions are able to diffuse through the cell membranes where they can distort the pH-equilibrium and transport systems of the pathogen.
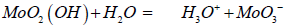
b) In addition to this mechanism also free radicals e.g. oxygen radicals and hydroxyl radicals are formed which result in a synergistic mode of action [30] (Figure 1).
c) A positive zeta potential has also been determined limited to a μm distance at the surface. This is reflected by an extraordinary fast eradication of microorganisms i.e. a reduction of 5 log 10 within 15 minutes documented by laser scanning microscopy [31].
d) Last not least paramagnetic Ions also contribute to the antimicrobial activity [32]. This technology is the only one which meets all the requirements described initially for prevention of Hospital acquired infections. The additives are water, detergent and alcohol insoluble and are fixed in a polymer or a coating where they are not eluted [33]. There is no induction of resistance, no allergenicity, the additives are nontoxic and are essential trace elements in the body! Permanent (>10 years) activity – including activity against microorganisms in biofilms has been documented Figure 2. Easy cleaning has been documented with water and detergents as microorganisms don’t adhere on acid surfaces. 1000 cleanings with water and a detergent did not impair the antimicrobial activity of this technology.
The technology is also active against microorganisms embedded in a biofilm! Microorganisms in a biofilm are hibernating and don’t take up anything from the outside. Therefore, all technologies which are based on incorporation of the antimicrobial agent into the bacterial metabolism are ineffective against microorganisms in a biofilm. Again, technologies which attack microorganisms from the outside also eradicate microorganisms in a biofilm, an important asset. However also technologies based on oxygen radicals alone are also not sufficiently active as these free radicals are not able to penetrate the biofilm. This has been documented for Titanium oxide. The antimicrobial technology of this technology is approved by the BPR of the EU as in situ generated biocides and is legitimately on the market. As these additives are not eluted to the surface, no toxicity is observed. Molybdenum is an essential trace element in the body as stabilizing molecule for several enzyme systems responsible e.g. for the elimination of sulfur in the body.
Figure 2:
(a) Investigation of activity by the JIS 25923 methode and
(b) The RODAC push plate method.

The antimicrobial activity and marketability of these in situ generated biocides has been documented by the Austrian ministry of environmental protection as rapporteur of the BPR of the EU. In the future no easy approval for “in situ generated biocides” is possible by ECHA. This opportunity expired September 1, 2018. This is also an additional favorable asset of the presently available technology. Additional technologies with limited usefulness or profound toxicity e.g. Ruthenium, Nicelous hydroxide have been propagated:
Four additives are available as in situ generated biocides with important additional properties:
a) Molybdenum oxide. Molybdenum is incorporated in thermoplastic polyurethane in use for antimicrobial ECG lead wires. The antimicrobial activity has been documented: Immersion in 109 CFU/ml for 6 hours shows no growth at the surface after application on blood agar plates. The duration of the antimicrobial activity is more than 20 years. The results of numerous external investigations are available. Additional application in push buttons, artificial leather with a blue grayish appearance has been endowed with molybdenum oxide. The advantage Molybdenum oxide is an inexpensive additive, readily available in unlimited quantities.
b) 5 % Oxygen deficient Tungsten blue oxide is in use for surfaces in permanent contact with water e.g. pipes faucets in hospitals. This can prevent the growth of legionella in faucets. The endowment of water heaters e.g. incorporated into enamel has also be investigated providing a permanent coating. Oxygen saturate tungsten yellow oxide shows limited antimicrobial activity.
c) The incorporation of Molybdenum into the zinc oxide crystal lattice results in a highly active white or - with submicron particles - transparent coating or paint. Zinc Molybdate has the broadest application for hospital furniture, for leather, textiles or artificial leather in numerous applications in public transportation, for office furniture in contact with different customers. The antimicrobial activity is very broad including a number of viral pathogens like bird flu, swine flu, influenza, Herpes, Epstein Barr virus [34-36]. Transparent coatings are available with particle sizes of the additives e.g. Zinc Molybdate of 0.20 μm (Lambda half) [33].
d) The incorporation of Molybdenum oxide into the tungsten crystal lattice provides additional antimicrobial features due to a strong zeta potential: The antimicrobial activity against bacterial pathogens is fast and includes fungi and molds (Aspergillus spp), the majority of viral pathogens like hepatitis B, C, COVID 19. Investigations of the antimicrobial activity against COVID 19 has been performed by the MSL laboratory: The test product received has achieved a 99.22 % reduction of feline coronavirus under the conditions stipulated. Documents MSL Laboratories, Gollinrod UK [37,38]. There is also a strong activity against algae providing antifouling surfaces for marine vessels [39].
e) All additives can be used as transparent coating or paints if applied as submicron particle sizes (lambda half) However great caution has to be exhibited to leave the delicate orthorhombic and monocline crystal structure intact. These particles with a size of 0.2 μm can be achieved by thermal fracturing.


