Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Bogatyrev Evgeniy and Kodkin Vladimir*
Received: August 03, 2022; Published: August 12, 2022
*Corresponding author: Weihao Zhu, College of Chemistry and Chemical Engineering, Henan Polytechnic University, Jiaozuo, 454003, China
DOI: 10.26717/BJSTR.2022.45.007237
To date, temperature-controlled phase-transfer catalyst, supported phase-transfer catalyst, and hydrogen bond phase-transfer catalyst are regarded as alternatives to traditional phase-transfer catalyst such as onium salt crown ether and polyether phase-transfer catalyst, have drawn significant attention from related scientists owing to their excellent properties, including nontoxicity, well-cycling performance, low cost, and facile synthesis. Especially, supported phase-transfer catalyst can not only overcome the poor solubility of heterogeneous systems came out but also can be reused many times and have been successfully applied in numerous technological fields. The influx of different novel recyclable phase-transfer catalysts is essential to overcome traditional phase-transfer catalyst’s large consumption, environmental pollution, difficulty in recycling, and high toxicity. Herein, this mini-review sum up recent advances in the organic synthesis of novel phase-transfer catalysts and their promising applications.
Keywords: Phase-Transfer Catalysis; Catalytic Mechanism; Dynamics; Green Synthesis
Abbrevations: PTC: Phase-Transfer Catalysis; ATRP: Atom Transfer Radical Polymerization; HBPTC: Hydrogen Bond Phase-Transfer Catalysis; PEG: Polyethylene Glycol; TBAB: Tetrabutylammonium Bromide
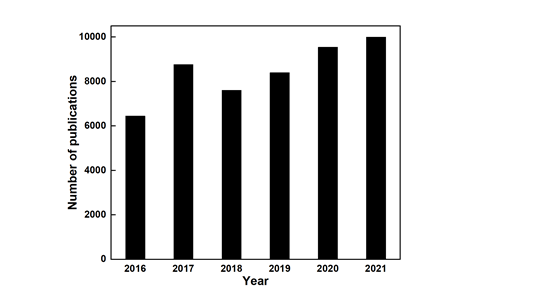
Phase-transfer catalysis (PTC) is a new technology developed in the early 1970s. It has been applied to various conventional processes in agrochemical, dyes, pharmaceuticals, polymers, perfumes, fragrances, and other fine chemical industries, mature organic synthesis technology for large-scale industrial application [1-3]. As obviously shown in Figure 1, numerous scientific literature studies related to phase-transfer catalysis have been gradually published since 2017. Designing novel phase-transfer catalysts and developing green synthesis processes will still be the focus of research in the field of chemical engineering for a period of time in the future.
Phase-transfer catalysis technology can speed up the reaction process under mild reaction conditions, improve product yield and selectivity, provide a method for overcoming the immiscibility of reactants in heterogeneous systems, and avoid expensive use [4-6]. Therefore, the phase-transfer catalysis technology has been rapidly developed, and remarkable results have been achieved in both laboratory and industrial applications.
However, phase-transfer catalysis also has the disadvantage that it is hard to separate the product from the organic phase [7-9]. To solve this problem, researchers have proposed a threephase transfer catalyst system. When the phase-transfer catalyst is insoluble in the organic or aqueous phase, a third phase can form, and once the third phase is created, the reaction rate increases dramatically [10-15]. Some common traditional phase-transfer catalysts are cyclic crown ethers [16,17], polyether [18-21], tertiary amines [22], etc. New supported phase-transfer catalyst materials include polymers [23-26], silica [27-29], magnetic nanoparticles [30-35], nanocellulose [36], etc. Thanks to the existence of the phasetransfer catalyst, the negative ions participating in the reaction have high reactivity, and the process operation is simplified. It has been successfully applied in various organic reactions. Although many novel phase-transfer catalysts appear every year, the continuous emergence of these novel phase-transfer catalysts has injected novel vitality into the field of organic synthesis.
Figure 1: The number of published literature studies according to the X-MOL search (keyword “Phase-transfer catalysis”).
Still these phase-transfer catalysts are difficult to put into industrial production and they are difficult to recover and consume. Therefore, some novel type of recyclable supported phase-transfer catalysts provides a sound way to resolve the above problems. Notably, the reusability of the novel phase-transfer catalyst gives economic viability to the industrial application of the novel green process [37]. As a result, in this minireview, first, the development process of novel phase-transfer catalysis is reviewed and discussed in detail in recent years. Then, we briefly study reaction mechanism types and kinetics of phase-transfer catalysts of phase-transfer catalysis. Eventually, we will offer several future outlooks and emphasis the significance of phase-transfer catalysis employed for promising catalytic applications.
Many organic synthesis reactions are easily carried out under homogeneous conditions, but not under heterogeneous conditions, especially in the two phases of water and organic solvent, reaction is incomplete, or even no reaction occurs, although stirring can increase the chance of mutual contact, the effect is not very obvious [38]. Generally, in order to promote its reaction, polar solvents are often used to make it a homogeneous reaction. Still, the use of protic solvents can cause solvation of the reactants, affecting the reaction speed, and the use of polar aprotic solvents can overcome the solvent. However, the price is high, and it needs to be carried out under anhydrous conditions. There are specific difficulties in recycling, so the emergence of phase-transfer catalysis technology has develop inevitable [39]. In the background of its original discovery, the term phase-transfer catalysis was used to refer to the reaction of ionic compounds and organic, water-insoluble substances in low-polarity solvents.
The concept of phase-transfer catalysis later became broader and involved the extraction of cations and even neutral molecules from one phase to another (usually an organic phase) with the help of a catalyst. In a phase-transfer catalytic system, the catalyst can be homogeneous (soluble in one or all solvents) or heterogeneous (bound on a solid support). Grafting traditional phase-transfer catalysts onto high molecular weight polymers is the most emerging novel type of recyclable phase-transfer catalysts in recent years, which greatly solves the problem of separation and recovery of phase-transfer catalysts. Compared with conventional phase-transfer catalysts such as onium salt and crown ether, novel phase-transfer catalysts have already attracted growing research attention in the matter of their magnetic properties, containing low toxicity, excellent cycling performance, high selectivity, and ease of surface modification or functionalization [40,41]. The following is the development process of novel phase-transfer catalysts in recent years, including temperature-controlled phase-transfer catalyst, supported phase-transfer catalyst, hydrogen bond phase-transfer catalyst.
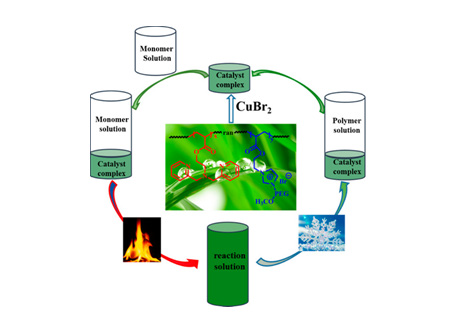
For temperature-controlled type phase-transfer catalyst, Bingjie Zhang, et al. [42]. designed a thermally regulated random copolymer PIL (PILL) with ionic liquid and atom transfer radical polymerization (ATRP) ligand side chains was synthesized to form macroscopic complexes with CuBr2 and used as an ATRP catalyst to establish thermally regulated phase separation catalysis (TPSC) system and further application. This novel TPSC system can realize the simultaneous recovery of transition metal catalysts and ligands by simply changing the polymerization temperature to room temperature. (As shown in Figure 2). Next year, Zhouyang Long, et al. [43]. simultaneously used graphitic carbon nitride (C3N4) and Ch5PMoV2 to establish an easy-to-recycle catalytic system that can realize the liquid-free phase of benzene, reductive aerobic oxidation to phenol. Temperature-controlled phase-transfer catalyst C3N4- Ch5PMoV2 exhibits high activity with 10.7% phenol yield with high turnover (TOF) and excellent reusability.
Figure 2: A thermoregulated phase-separated catalysis (TPSC) system schematically illustrated. Reprinted with permission from ref. [42].
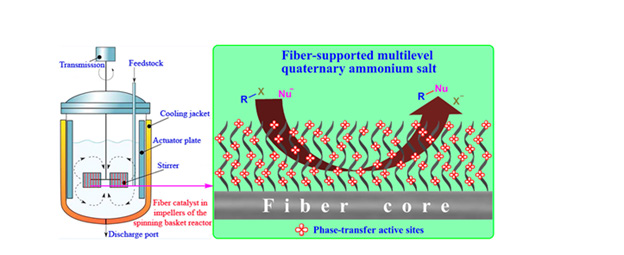
In 2021, Zhi Yang, et al. [44] have been used to hydrogenation of phenolic compounds and lignin oil by using an efficient binary catalyst of tungstic acid (H2W4) and Ru/C under temperaturecontrolled phase-transfer catalysis deoxygenation (HDO). This novel temperature-controlled type phase-transfer catalyst system has the advantages of simultaneous recovery of catalyst and ligand, easy in-situ separation, recovery only by changing the temperature, high catalyst activity, and high recovery efficiency. Temperaturecontrolled phase-transfer catalysis is no longer limited by the water solubility of substrates and has attracted extensive attention due to its innovative theory and attractive application prospects. For supported phase-transfer catalyst, in 2018, X.-L. shi [45] research group formed a novel type of fiber-supported phase-transfer catalyst, polyacrylonitrile fiber supported multi-level quaternary ammonium salt and explained the multi-level quaternary ammonium salt through the phase-transfer catalytic mechanism.
The catalytic effect created by the amphiphilic cyano group on the polymer skeleton of the fiber has excellent recyclability, and there is no apparent loss of catalytic performance even after repeated use 15 times. (As shown in Figure 3). Depending on the superiority of PANF, and it is attractive to imitate the spinning basket reactor with fiber catalyst in its impellers for actual productions. Plant trials of fiber catalysts in spin basket reactors are underway. In 2019, Ji-Chuang Shen and Wei-Ling Jiang [46] positively synthesized three COFs-containing crown ethers (CE-COFs) and rooted various secondary rings (12-crown-4, 15-crown-5, and 18-crown-6). CE-COFs display excellent performance as phasetransfer catalysts (PTCs) in nucleophilic substitution reactions such as esterification, etherification, and cyanation. A variety of crown ether-functionalized p-triphenylenediamine monomers were condensed with 1,3,5-triphenylene benzene under solvothermal conditions to obtain three different crown ethers.
Figure 3: Polyacrylonitrile fiber supported multi-level quaternary ammonium salt schematically illustrated. Reprinted with permission from ref. [45].
In 2020, Mehdi Fallah-Mehrjardi and Mahdieh Shirzadi [47] ,etc. synthesized a novel nano-magnetic carrier organocatalyst (MNP@PEG-ImOH) in the aqueous medium. They efficiently synthesized 2-amino-3-cyano-4H-Pyran, the catalyst can be easily separated from the reaction mixture using an external magnet and can be reused in subsequent runs without loss of activity. Supported phase-transfer catalysts have significant advantages in recovery, catalytic efficiency, firmness of catalyst loading, service life, and price. Therefore, it has excellent application potential and can be widely used in various organic synthesis reactions and largescale industrial production. For hydrogen bond phase-transfer catalyst, Lin Hu, et al. [48] first straight catalytic asymmetric synthesis of γ-amino ketones was accomplish by the progress of a greatly diastereoselective and enantioselective C−C bond-forming umpolung reaction of imines and enones under the catalysis of a novel cinchona alkaloid-derived phase-transfer catalyst in 2016.
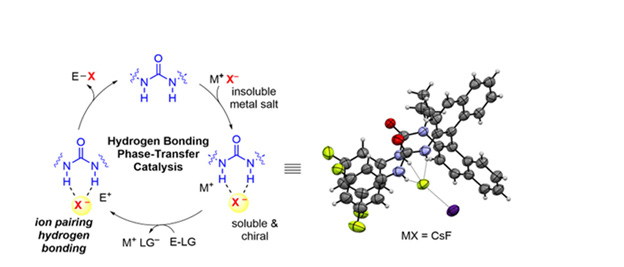
The following year, Duan, et al. [49] reported a quinine-derived bifunctional thiourea phase-transfer catalyst catalyzed ketone imide nitro-Mannich. It was also the first phase transfer catalyzed by ketone imines nitro-Mannich reaction. Then Maciej Majdecki, et al. [50] examined this kind alkaloids in the alkylation of glycine derivative, under phase-transfer catalyst conditions. High reaction yield and high enantioselectivity are possessed by this phasetransfer catalyst. A series of phase-transfer catalysts with multiple hydrogen bonds have been developed using cinchonine as a chiral source, which has been successfully applied in different asymmetric reactions. In 2022, Gabriele Pupo and Véronique Gouverneur [51] investigated hydrogen bond phase-transfer catalysis (HB-PTC), a novel concept in PTC originally designed for alkali metal fluorides but providing enantio opportunities beyond selective fluorination. (As shown in Figure 4). In the same year, Jimmy Wang, et al. [52] studied in detail the application of hydrogen bond phase transfer catalysis (HB-PTC) in the enantioselective azide reaction of sodium azide. Collectively, this study highlights the potential of hydrogenbond phase transfer (HB-PTC) catalysis beyond fluorination and as a general mode of activation as a reagent for a large number of alkali metal salts in asymmetric. Going forward, the infinite choices of diversifying hydrogen bond donors, inorganic salts, and electrophiles herald a new era in phase-transfer catalysis. Whether the abundant inorganic salts with significantly higher lattice energies than those studied to date, such as CaF2, can be considered as nucleophiles, remains an open question that could be solved by a synergistic phase-transfer catalysts approach.
Figure 4: Hydrogen bond phase-transfer catalysis (HB-PTC) schematically illustrated. Reprinted with permission from ref. [51].
The phase-transfer catalysis mechanism was first proposed in 1978. Under the action of phase-transfer catalysts, a large number of reactions that were initially slow or required severe conditions can now be completed in high yields under mild conditions [53- 55]. Most of the phase-transfer catalytic reaction mechanisms focus on the discussion of nucleophilic reactions, including several successive stages. To fully grasp the technology in this area, it is essential to have a deep understanding of the influencing factors of each stage. In general, phase-transfer catalysis reactions include two basic steps:
1. Transfer a species from one phase to another, and
2. The transferred species reacts with another species.
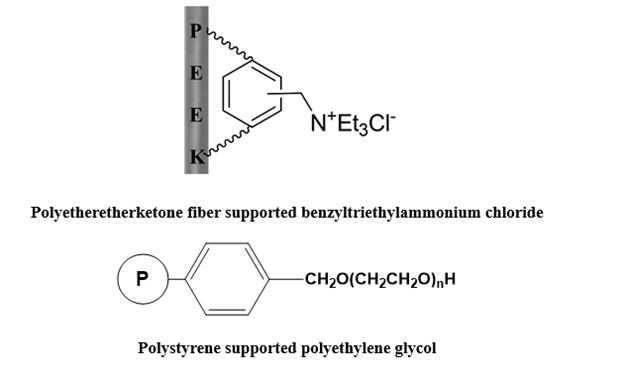
When the phase-transfer catalyst is insoluble in neither the organic nor the aqueous phase, the phase-transfer catalyst will be present in the third phase so that both reactants are transferred into the third phase, where the two reactants are dissolved in each other, and the concentration is very high, which can show high reactivity, so that the reaction speed is obviously improved. In general, from the perspective of phase-transfer catalysis, there are “extractive” phase-transfer catalysis and “interface type” phasetransfer catalysis. The extraction mechanism shown in Figure 5 is mainly used in alkylation, esterification, etherification and easy displacement reactions in which nucleophiles are transferred to the organic phase through soluble catalysts in the organic phase. The interfacial mechanism shown in Figure 6 is applied to carbanion reactions, carbene reactions, condensation of polymerization, and c-alkylation of active methylene compounds [56]. With onium salts (benzyl triethylammonium chloride), and polyether (polyethylene glycol), two phase-transfer catalysts are used as examples to explain the mechanism of phase-transfer catalysis:
Benzyltriethylammonium chloride [57] can be used as a phasetransfer catalyst because of its amphiphilic groups in its structure, which are both soluble in the organic phase and soluble in water. For example, in the synthesis reaction of p-nitroanisole, methanol and sodium hydroxide react firstly to generate the nucleophile CH3ONa. Then the catalyst and the nucleophile in the aqueous phase form the ion pair Q+CH3O-, which makes the original insoluble in the organic phase. The nucleophile is dissolved in the organic phase, and the target product p-nitroanisole is formed with the reactants in the organic phase. After the reaction, the leaving group is combined with Q+ to create an ion pair, which is returned to the water phase, and the cycle is realized transfer between the two phases. Polyethylene glycol (PEG) [58] can also be used as a phase-transfer catalyst because of its zigzag shape in the absence of water and a curved chain shape in the presence of water. The curved-chain polyethylene glycol has the ability to complex metal ions. After complexation, it has a positive charge, combining with nucleophilic particles to form ion pairs, which are extracted into the organic phase. In the organic phase, the polyethylene glycol is again serrated, and the metal ions are separated from the polyethylene glycol and return to the aqueous phase. After the nucleophilic particle reacts with the organic phase, the polyethylene glycol returns to the aqueous phase, then takes the form of a curved chain, and the cycle plays the role in phase-transfer catalysis.
The function of the phase-transfer catalyst is to bring the reactant into the organic phase when an ion pair is formed with the reactant in the aqueous phase in the two-phase system, thereby realizing or accelerating the process of the reaction. The essential characteristics of phase-transfer catalysts are that the catalyst must have the ability to transfer the reacting anions into the organic phase, perform a nucleophilic attack on the organic substrate, and make the binding of cations and anions loose enough to allow high reactivity in the organic phase rate. Request the molecular structure of the catalyst should contain a positive ion part, so that it can form ion pairs Q+Y-, Q+X- with the negative ions of the reactant, and secondly, Q+ must have a certain amount of lipophilic. Common phase-transfer catalysts are: onium salt phase-transfer catalysts, crown ether, and polyether phase-transfer catalysts, and supported phase transfer catalysts.
Onium salt phase-transfer catalysts are the earliest widely used phase-transfer catalysts, including quaternary ammonium salts, quaternary phosphorus salts, and sulfonium salts [59,60]. The phase-transfer catalysts of onium salts are composed of three parts: the central atom, the substituent on the central atom. The negative ion, and the catalytic activity are related to these three parts. In order to make the quaternary ammonium salt cation have good lipophilicity and good hydrophilicity, the total carbon atoms of the hydrocarbon group should be greater than 12, generally about C12-C25 works well. For example: benzyltriethylammonium chloride (TEBA), tetrabutylammonium bromide (TBAB), trioctylmethylammonium chloride (TCMAC), etc. The higher the symmetry of the quaternary ammonium salt, the tighter the positive charge is shielded, and the better the catalytic effect of the phase-transfer catalyst. The structure of quaternary phosphate salt is similar to that of quaternary ammonium salt, and the catalytic principle is also the same. Although the price of quaternary phosphate salts is slightly more expensive than that of quaternary ammonium salts, quaternary phosphate salts are more stable to strong alkali and heat as phase-transfer catalysts than quaternary ammonium salts. Therefore, quaternary phosphate salts are gradually used.
Crown ether CH2CH2O structural units are compounded into macrocyclic molecules, which can be divided into many types according to the number of atoms on the ring and the number of oxygen atoms in the ring. The larger the ring, the larger the inner pore size of the molecule [61,62]. Crown ethers are often used in liquid-solid reaction systems. The main factors affecting the catalytic effect are the pore size of crown ethers and the substituents on the ring. The common ones are: 12-crown-4, 15-crown-5, 18-crown-6, 21-crown-7, etc. Crown ether phase-transfer catalysts generally have higher lipophilicity than onium salt phase-transfer catalysts, and their catalytic ability is stronger than onium salts (Figure 7).
Polyether phase-transfer catalysts include polyethylene glycol, polyethylene glycol fatty ether, polyethylene glycol alkyl phenyl ether, etc., and have the advantages of low price, good stability, and easy synthesis. Open-chain polyethylene glycol also has multiple CH2CH2O structural units, which act like crown ethers as phasetransfer catalysts. For polyethylene glycol phase-transfer catalysts with different molecular weights, as long as the number of units of CH2CH2O is the same, the catalytic activity is basically the same. The catalytic effect of polyethylene glycol mainly depends on the number of units of CH2CH2O, that is, the number of holes formed by the CH2CH2O unit chain. Therefore, as the molecular weight of polyethylene glycol increases, its catalytic activity also increases accordingly.
Supported phase-transfer catalysts are the most extensively used and potential phase-transfer catalysts. The supported catalyst combines the catalytic performance of the catalyst with the characteristics of the carrier material, which simplifies the separation operation steps while maintaining or improving the catalytic activity, thereby facilitating the recycling of the catalyst. Supported phase-transfer catalysts, which are insoluble in water, acids, bases, and organic solvents, can be recovered by simple filtration after the reaction, can be reused, and are very suitable for continuous industrial production [63-65]. Support types include organic supports (polystyrene resin, chlorinated polyethylene) and inorganic supports (silica gel, alumina).Typical three-phase phase transfer catalysts include immobilized ammonium salt, immobilized polyethylene glycol, immobilized crown ether, etc. For example: polyether ether ketone fiber supports benzyl triethyl ammonium chloride, and polystyrene supports polyethylene glycol (Figure 8).
Figure 8: Enhanced scale of “complex” sections of diagrams: initial signal (blue); signal after processing (red), “F” - fetal cardiocomplex, “M” - pregnant cardiocomplex.
Phase-transfer catalysts are either soluble in one of the two phases or insoluble in the third phase, thereby significantly increasing the reaction rate. Adding a small amount of phasetransfer catalyst has the beneficial effect of accelerating the rate of reaction between the components present in the two immiscible phases. The activity of phase transfer catalytic reaction is controlled by many factors such as catalyst activity and dosage, solvent type, stirring speed, and temperature and so on [66].
The phase-transfer catalyst has an excellent catalytic effect on the reaction, and the reaction is also very sensitive to the change in the catalyst concentration. Under other conditions being the same, with the increase of the catalyst concentration, the number of catalyzed active sites increases, and the conversion rate also increases. The rise in the reaction rate is proportional to the amount of catalyst, which often occurs in some lipid hydrolysis reactions and substitution reactions. For different reaction systems, the amount of catalyst usually varies from a few mole percent to several moles. However, for some polymer solid-supported phasetransfer catalysts, excessive phase-transfer catalysts may also lead to the adsorption of reactants or products to the catalyst’s surface, increasing the amount of organic matter adsorbed, thereby affecting the conversion of the reaction.
Most organic reactions need to consider the influence of solvent on the reaction. For example, benzene and benzene carbon tetrachloride are often used as organic solvents. Benzene as the reaction medium is huge, probably because benzene is an aromatic compound, and carbon tetrachloride is an aliphatic solvent. For the difference in conversion rate between trans and carbon tetrachloride, the solubility of the substance in the solvent is different, which leads to differences in the conversion of the target product. Low-boiling fluorine-containing solvents (e.g., chloroform, dichloromethane, 1,2-dichloroethane) are the best, not only have higher extraction capacity for salts, but also are cheap and easy to recycle.
The size of the interface area controlled by the stirring rate will affect the extraction equilibrium position of various anions and cations provided by the phase-transfer catalyst. As the stirring speed increases, due to the enhancement of mass transfer, solubility is affected by stirrer speed, leads to the probability of molecular collision during the reaction increases, and the yield of the reaction increases.
The temperature has an insightful effect on the transformation of the phase-transfer catalytic reaction. As the temperature grows, the kinetic energy of the molecules increases, which further increases the rapidity of the availability of reactant molecules in the reaction. Therefore, increasing the temperature will increase the phase-transfer catalytic reaction transform. However, if the temperature is too high, some groups will be decomposed and damaged, resulting in a decrease in the reaction yield. Therefore, for different phase transfer catalytic reactions, it is meaningful to find a suitable and optimal reaction temperature for the catalytic reaction.
For instance, the reaction formula of p-nitrochlorobenzene, methanol, and sodium hydroxide under the action of phase-transfer catalysts is [67-69] (Figure 9): The reaction of p-nitrochlorobenzene and sodium methoxide is a two-step SN2 reaction. Add a phasetransfer catalyst (e.g., fiber-supported benzyltriethylammonium chloride phase-transfer catalyst Q) to the reaction medium, and the reaction begins. After Cl- and CH3O- undergo ion exchange, CH3O- is transferred from the aqueous phase to the phase-transfer catalyst surface, and p-nitrochlorobenzene undergoes a substitution reaction with CH3O- to complete the phase-transfer catalysis process. The following are possible reaction steps:
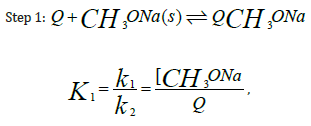
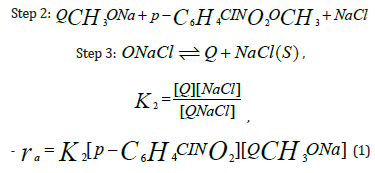
Substituting [QCH3 ONa] =Q1[Q] in equation (1) results in
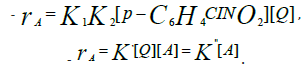
Writing the equation in terms of conversion and integration results in
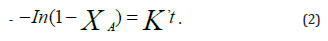
Where 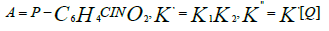
In this minireview, recent developments on applications of phase-transfer catalysts are overviewed and discussed in detail, allowing us to go one step further and look at phase-transfer catalysts. At present, phase-transfer catalysis is widely used in drug synthesis, pesticide synthesis, and the chemical industry. It also has applications in chemical modification, polycondensation, and free radical condensation of polymers, it can be seen that the application prospect of phase-transfer catalysis is good. Not only that, temperature-controlled phase-transfer catalysis, reverse phasetransfer catalysis, and asymmetric phase-transfer catalysis have also been gradually applied to a range of organic synthesis reactions, but the application of phase-transfer catalysts in industrial production lines is still not very mature and the development in this direction needs to be further strengthened. Polymer solid-supported phasetransfer catalysts are the most extensively used, combining the advantages of high molecular polymers and phase-transfer catalysts, with high mechanical strength, non-toxic and non-polluting, which undoubtedly provides a practical solution to the problem of difficult separation of phase-transfer catalysts feasible path. In addition, in the future, the application of microwave technology and ultrasonic waves in phase-transfer catalysis reactions should also develop more rapidly, because not only the conditions of the reaction are improved, but also the yield of the reaction is improved, and the possibilities of possible organic synthesis reactions are expanded scope. In conclusion, phase-transfer catalysis combined with new synthetic methods is becoming the mainstream in the research field after years of development, and also promotes the development of environmentally friendly catalysis technology, which has produced a wide range of basic chemistry, fine organic synthesis, and biochemical science. The application prospect of the phase-transfer catalyst makes the development prospect of the phase-transfer catalyst brighter.


