Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ashok Chakraborty1* and Anil Diwan2
Received: July 27, 2022; Published: August 10, 2022
*Corresponding author: Ashok Chakraborty, All Excel, Inc., West Haven, CT, USA
DOI: 10.26717/BJSTR.2022.45.007229
SARS-CoV-2 is one of the known beta family members of human coronaviruses, like HCoV-HKU1, SARS-CoV-2, MERS-CoV and SARS-CoV. SARS family viruses cause severe respiratory disease in humans. SARS-CoV-2 has recently considered as a pandemic with over 100 M confirmed cases and over 1.5 M death worldwide. Vaccines and antibodies appeared as record pace. However, generation of variants has already been demonstrated as an escape of virus from a particular vaccine therapy. Therefore, a broad-spectrum anti-CoV therapies should be emphasized over antibody therapy.
Keywords: COVID-19; SARS-CoV-2; Vaccine; Antibodies; Biopolymer; NV-CoV-2; Antiviral
Abbrevations: CoV: Coronavirus; CsA: Cyclosporin A; DMV: Double Membrane Vesicles; FDA: Food And Drug Administration; hAPN: Human Aminopeptidase N; HAT: Human Airway Trypsin-Like Protease; ORF: Open Reading Frame; SARS: Severe Acute Respiratory Syndrome; IFN: Interferon; IBV: Infectious Bronchitis Virus; hDPP4: Human Dipeptidyl Peptidase 4; MERS: Middle East Respiratory Syndrome; NHP: Non-Human Primate; TMPRSSII: Transmembrane Protease; HCoV: Human Coronavirus; RBD: Receptor Binding Domain; hACE2: Human Angiotensin Converting Enzyme 2; RNA: Ribonucleic Acid
Mature human coronavirus is a double membrane compartment particle, and one of them is SARS-CoV-2 is a recent threat to human life, causing COVID-19 [1]. SARS-CoV-2 infects the human through binding to the host cell surface angiotensin-converting enzyme 2 (ACE2) receptor [2,3]. Following infection, the majority of victims develop detectable serum antibodies to the viral spike protein [4,5], however, mild infection may not develop such a countable number of neutralizing antibodies [6,7], and in addition, the neutralizing activity of those antibodies is maintained for up to six to eight months, only [8-11]. Studies have shown that SARS-CoV-2-specific CD4 and CD8 T cells response in patients who had recovered from COVID-19 and in individuals who had received an investigational COVID-19 vaccine [12-14]. However, the life of neutralizing antibody that generated after the first infection is short and becomes undetectable during the second infection [15]. Further, the reinfection with SARS-CoV-2 was detected sporadically [16-22].
The scientific community at large and regulatory efforts have remained focused on
(a) Vaccines
(b) Antibodies and
(c) Redevelopment of pre-existing drugs as antivirals for COVID-19 therapy [23].
The strong government support led to the emergency approval of two different antibody drugs, one from Regeneron, and one from Eli Lilly in the fastest ever drug development timeframe. Both developed against the original 2019-nCoV-Wuhan variant, one by Pfizer-BioNTech, and one by Moderna.
mRNA vaccines are good to raise both cellular and humoral immunity but not restricted by MHC. In addition, since mRNA does not interact with the genome it can be considered as a safe vector to carry information. Further, mRNA vaccines, with respect to development, also offer maximum flexibility in protein expression [24]. BioNtech and Pfizer, both have already announced the results of their phase 3 clinical trial with mRNA vaccines on COVID-19 patients [25]. Moderna, another company also released their phase 3 clinical results during the same time [26]. In both the cases, efficacy in preventing SARS-CoV-2 infection was 95% and 94.5%, respectively. Besides, a small German start-up and the American Pharma jointly have developed a vaccine, BNT162b2, while the Cambridge-based biotech company in collaboration with the National Institutes of Health, developed mRNA-1273 vaccine against SARS-CoV-2 infection. Detailed efficacy of those vaccines, however, are not known yet.
SARS-CoV-2 virus has 12 structural proteins and 15 nonstructural proteins (NSPs) and accessory proteins. The structural gene region encodes spike protein (S), envelope (E), membrane (M), and nucleocapsid protein (N) [1,27]. The spike (S) protein of SARS binds with the host cell surface angiotensin-converting enzyme 2 (ACE2), and then uses the host transmembrane serine protease-2 (TMPRSS2) to do the priming of the S protein for efficient fusion and cellular entry to the cell [1,28] (Figure 1). “M” protein is embedded in the envelope and maintain the shape of the virion envelope. “E” protein is a small polypeptide important for CoV infectivity. “N” protein binds to the S protein, makes up the helical nucleocapsid and does the insertion of fusion peptide (FP), which are required for virus– host membrane fusion [29-32].
Figure 1: SARS-CoV-2 genome and strategies for stabilizing the spike protein. A cartoon picture of SARS-CoV-2 virion with structural proteins (spike S), membrane (M), nucleocapsid (N) and envelope (E)) were shown.
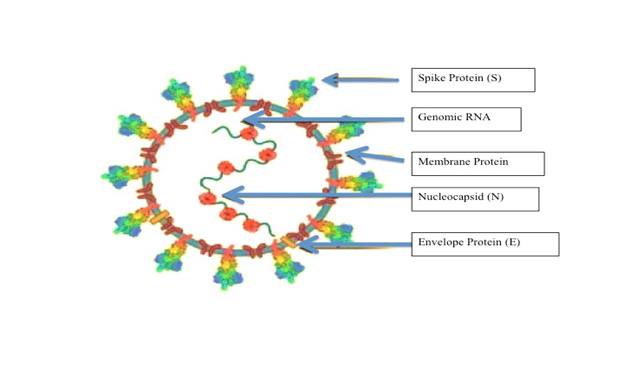
S protein is composed of two subunits (S1 and S2), it mediates both virus-host attachment via its receptor-binding domain (RBD) [33]. Therefore, it could be another target of neutralizing antibodies, which are most closely associated with protective responses against viral infection in humans.
Mammals including pangolins, pets (dogs and cats), and Cricetidae family members may be used for determining the key residues for association with S protein from SARS-CoV and SARSCoV- 2. These may provide the additional information regarding invasion and pathogenesis of the virus, which may support the vaccine design [34]. In the course of virus epidemics, the ability to adapt to external pressure is an important factor affecting the spread of the virus. Mutation or recombination of S-protein gene at position 614 from aspartate (D) to glycine (G614) makes it difficult to develop neutralizing antibodies and/or vaccines against the viral proteins, and finally promotes transmission efficiency and higher fatality rate [35]. To prevent the disease effectively, combinations of different mAbs that identify different epitopes on the SARS-CoV-2 S surface protein can be assessed first to neutralize a wide range of mutant viruses [36].
Peptides derived from heptad repeat 1 (HR1) or heptad repeat 2 (HR2) of the S2 subunit from SARS-CoV-2 inhibit viral fusion with the target cells. Moreover, nAbs (neutralizing antibodies) against S2 protein have been reported to target the S2 subunit of SARSCoV- 2 suggesting the S2 subunit could be a better vaccine target for COVID-19 therapy [19,29,37,38]. However, the membraneproximal end of S2 subunit contains extensive N- glycan which makes it less accessible for immune recognition than the S1 subunit [39,40]. In fact, rabbits immunized with S2 protein of SARS-CoV-2 expressed much lower nAb titres compared to immunization with the S1 subunit or RBD proteins [41]. However, having the conserved sequence of the S2 subunit between the virus species, the cross-reactivity of the antibodies and CD4+ T cells are expected, and may suggest a potential target for a universal CoV vaccines [18,19,42,43].
CoV M and E proteins, unlike S protein, have small ectodomains and small molecular sizes, therefore becomes poorly immunogenic for humoral responses [44,45] (Figure 1). However, the sequence identity of M or E proteins among hCoV viruses [46] may improve cross-protection by T cell responses if included in a SARS-CoV-2 vaccine. hCoV N protein is most abundant viral protein and also highly immunogenic. [47]. It contains T-cell epitopes and is a major target for antibody responses [48]. N- specific CD4+ T cell epitope isolated from Venezuelan equine encephalitis virus showed complete protection against SARS-CoV infection [49]. However, Nprotein antibodies cannot protect from SARS- CoV-2 infection in a mouse model [50]. Furthermore, no N protein-based vaccine for COVID-19 is reported, so far.
In fact, the receptor binding domain of SARS viruses binds to the host cell via its receptor binding motif (RBM) [51-53]. Though the surface of the S protein is generally shielded by glycans from antibody recognition, but it is not case with RBD, therefore makes it immune-dominant [54]. Most SARS- CoV-2 neutralizing antibodies (nAbs) is therefore effective in inhibiting virus attachment to the cell [55-66]. The RBD also express the epitopes for T cell responses [46,67-69]. A recent report showed that an RBD-based DNA vaccine are immunogenic and protects NHPs from COVID-19 [70]. An RBDbased mRNA vaccine is currently in phase I trials in China. Despite of all the above facts the use of RBD vaccines is limited because of its low immunogenicity due to small molecular size and mixed structural forms of RBD. Recently, to address these limitations, a dimeric form of the RBD antigens is being made for using against SARS-CoV viruses [71]. BioNTech/Pfizer already reported an mRNA vaccine prepared from the RBD-trimer, BNT162b, which showed an induced nAbs and TH1 cell- biased responses [72,73]. Vaccination with N-terminal domain (NTD) express nAbs and NTD-specific T cell responses. However, the above vaccines though successfully tested with MERS-CoV-mouse model, but nothing is reported for COVID-19, yet [74].
Adenovirus Cassette: Adenoviral vectors are the new COVID-19 vaccine frontrunners. CanSino’s adenoviral vector vaccine made it into human trials in China in March ‘20. Later that month, J&J make up to 1 billion doses of its vaccine. Vaccine raised against human adenovirus type 5 (Ad5) expressing the full length of S protein has recently been developed in China and now entered in phase-III clinical trial for trying against COVID-19 [75,76]. Ad- Chimp used by Oxford (A-Z) to COVID. In 2012, the Oxford group developed its own chimpanzee-derived vector, dubbed ChAdOx1, based on an adenovirus discovered in chimpanzee feces. A single dose of ChAdOx1 nCoV-19, an investigational vaccine against SARSCoV- 2, has protected six rhesus macaques from pneumonia caused by the virus, according to National Institutes of Health scientists and University of Oxford collaborators. The findings are not yet peer-reviewed but are being shared to assist the public health response to COVID-19. Based on these data, a Phase 1 trial of the candidate vaccine began on April 23 in healthy volunteers in the United Kingdom. Oxford University has entered into a partnership with UK-based global biopharmaceutical company AstraZeneca for the further development, large-scale manufacture and potential distribution of the vaccine [77]. The second, being developed in the UK, uses recombinant chimpanzee adenovirus, ChAdOx1 [78,79].
Live attenuated virus (LAV) vaccines have been a reliable means of inducing effective long-term immunity against various specific viral pathogens, such as polio and measles. These are developed by passaging the pathogen under heterogeneous conditions. The weakened form of the virus cannot replicate easily within human cells. This allows the virus to generate an immune response but not to establish a productive infection. The mutant enzymes may be temperature-dependent in their activity. Alternatively, the structure of the non-structural protein (NSP) may be altered by temperature, hindering complex formation with other NSPs and therefore preventing viral replication. A50-18 thus isolated as a novel live attenuated vaccine candidate for the prevention of COVID-19 because they have less pathogenicity but retained the immunogenicity [80].
The coronavirus disease 2019 (COVID-19) pandemic disrupted our lives and our healthcare systems and accounted for millions of illnesses across the globe ranging from mild to severe to deadly. COVID-19 vaccination is a critical tool in stopping this pandemic. There are several COVID-19 vaccines that are in the late stages of development. Currently, two vaccines have been authorized for use by the U.S. Food and Drug Administration and recommended by the Centers for Disease Control and Prevention. These vaccines are designed to teach your body’s immune system to recognize and fight off the virus that causes COVID-19. Therefore, COVID-19 vaccines are a critical tool in stopping the pandemic, resuming normal life, and protecting ourselves and others from this disease.
A. Psychological comfort
B. May reduce severity of diseases even from a variant.
C. However, increase of ADE cannot be neglected. In case of SARSCoV- 1, ADE was demonstrated in animal model.
D. Vaccines are protective if given prior the exposure. However, the virus is constantly mutated.
Variants of SARS-CoV-2, a new virus having one or more mutations that differentiate it from the wild-type already circulating among the general population. Several new variants, most notably B.1.1.7, having a large number of mutations are found in US and UK [81,82]. In South Africa, another variant of SARS-CoV-2 designated as B.1.351 has emerged independently of B.1.1.7 [83]. Preliminary evidence from non-peer-reviewed publications suggests that the Moderna mRNA-1273 vaccine currently used in the US may be less effective against this variant, but additional studies are needed [84]. Similarly, in Brazil, P.1 variant with 17 mutations including three in RBD of the S protein (K417T, E484K, and N501Y), are reported [85,86]. Failure of vaccines and antibodies are therefore certain, the only question is how long it will be before the original vaccines become completely useless. Replacing current vaccine with a new vaccine, would be an endless game of chasing a rapidly changing epidemic nature of the disease that would be both costly and would be substantially non-responsive.
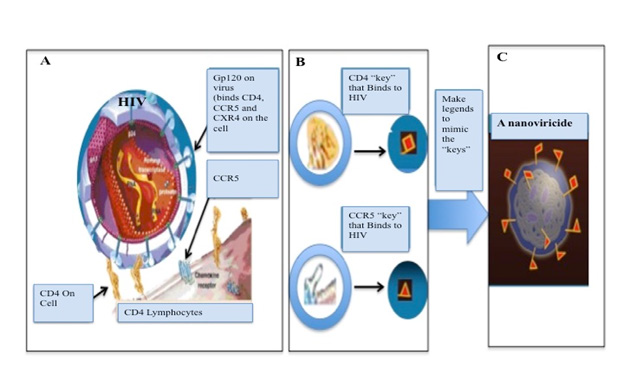
Attention needs to be focused on broad-spectrum antiviral therapeutics to minimize the possibilities of virus variants to escape from the drug effects. If such an effective drug is available, vaccines that require constant changes would not be needed. NanoViricides, Inc. (Shelton, CT, USA) recently has developed a technology to have a broad spectrum two arms antiviral theraputics. The drug can directly attack the virus and disable them to infect the human cells, and simultaneously can blocks the reproduction of the virus inside the cell. Together, this double-arm effect would result in a cure. Additionally, the Nanoviricides® platform technology is based on biomimetic engineering that copies the features of the human cellular receptor of the virus. No matter how much the virus mutates, all virus variants bind to the same receptor in the same fashion. In fact, the later variants bind to the receptor more effectively, in general. Thus, if these features of the cellular receptor are appropriately copied, the resulting Nanoviricide drug would remain effective against current and future variants of a virus. In brief, we have developed a “Venus-FlyTrap” for virus particles which is designed with such a unique concept that a when a virus encounters our Nanoviricide “Venus-FlyTrap” thinking it is binding to its target cell receptor(s), and would get engulfed and destroyed in the process (Figure 2). Modification of chemistry of the virus binding ligand portion of this nanomedicine we can add broad-spectrum quality to attack a different virus. This allows for rapid design & construction, synthesis, manufacture, and testing of novel drug candidates against an emerging outbreak such as the SARS-CoV-2 that has now become pandemic.
Figure 2: Nanoviricide represents a cell Mimic.
• A, B: HIV particle binds to cell via CD4 and a CCR5 and CXR4 present on the cell.
• C: A nanoviricide “looks like” a human cell to the virus.
• Nanoviricide is a small particle to circulate in the body, but big enough to latch on to the virus and to wrap it around.
Anti-coronavirus effectiveness of our Nanoviricide (NV-CoV-2, a broad-spectrum antiviral regimen), in cell culture studies using NL- 63 and 229-E CoVs. NL63 uses the same cell surface receptor ACE2 (angiotensin converting enzyme-2) as SARS-CoV-1 and- 2, while CoV- 229-E uses human amino-peptidase N (hAPN) cell surface receptor for binding. In both the cases NV-CoV-2 is effective in proving its broad-spectrum nature as antiviral therapeutics. Furthermore, NV-CoV-2 showed much more effectiveness than favipiravir (an RNA polymerase inhibitor) against two different coronaviruses in this study [87-89]. Safety and toxicity of nanovitricide drug candidates were studied in an animal model, at three different dosages (low, medium, and high) with vehicle as control. The tested drug candidates were safe and well tolerated [90,91]. There were no clinical signs of immune or allergic reactions noticed. Further, gross histology study on post-mortem no observable changes in any organs were noticed. The only reportable changes observed were, in the high dosage groups of two of the three drug candidates tested, associated with the non-absorption of water, in the colon. This is consistent with the clinical observation of very loosened stools in the same groups.
We have previously reported that Nanoviricide (NV-CoV-2) drug candidate have shown strong effectiveness in a lethal lung infection model in rats using a coronavirus (NL-63) that uses the same ACE2 receptor as SARS-CoV-2 which causes COVID-19 [92]. In those previous reports with lethal direct-lung-infection model efficacy study, animals in all groups developed lung disease which later led to multi-organ failures, a clinical pathology resembling that of the SARS-CoV-2. Reduction in loss of body weight at day 7 was used as the primary indicator of drug effectiveness. Rats were infected directly into lungs with lethal amounts of hCoV-NL63 virus particles and then different groups were treated separately with five different nanoviricides drug candidates, remdesivir as a positive control, and the vehicle as a negative control. The treatment was intravenous by tail-vein injection once daily for five days, except in the case of remdesivir wherein it was by tail-vein injection twice daily. In that efficacy study, animals treated with the five different nanoviricides showed significantly reduced body weight loss. The body weight loss in female animals ranged from only 3.9% to 11.2% in the different nanoviricide-treated groups, as compared to 20% in vehicle-treated control group, and 15.2% in a remdesivir treated group (n=5 in each group) [93-106].
The body weight loss in male animals ranged from 8.0% to 10.9% in the different nanoviricides-treated groups, as compared to 25% in the vehicle-treated control group, and 18.6% in remdesivir treated group (n=5 in each group). Smaller numbers mean less loss in body weight compared to starting body weight in the group, and indicate greater drug effectiveness. The strong effectiveness of nanoviricide drug candidates in the lung-infection model is consistent with the effectiveness observed in cell culture studies against infection of both hCoV-NL63, which was used in the efficacy study, and hCoV- 229E, another circulating coronavirus that uses a distinctly different receptor, namely APN (aminopeptidase). Prior to filing for human clinical trials, we plan on conducting studies, towards clinical candidate selection, to further determine the effectiveness against SARS-CoV-2, perform additional drug development studies as may be necessary, and request a pre- IND Meeting with the US FDA for regulatory guidance. Taken together, our approach and results give a strong confidence that: (a) our broad-spectrum anti-coronavirus drug candidates would most likely be effective against SARS-CoV-2, and (b) the potential mutations in the virus are unlikely to enable it to escape from these drug effects.
The study received funding from Nanoviricides, Inc., and properly acknowledged, however the funder had no role in the study conception, design, execution, analysis and decision to publish.
We acknowledge Nanoviricides for funding the project, and Julia Shiapong for her secretarial help and Ms. Bethany Pond for editing the manuscript.


