Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Dumitrache Marieta1*, Lascu Rodica2 and Cioboata Mihai Luca3
Received: July 18, 2022; Published: July 27, 2022
*Corresponding author: Dumitrache Marieta, Ophthalmology Clinic, University of Medicine and Pharmacy Bucharest, Romania
DOI: 10.26717/BJSTR.2022.45.007190
NF1 is a genetic, multisystemic neuroectodermal disembryoplasia in which the spontaneous or inherited mutation is located on the long arm of chromosome 17 q position 17q 112. NF1 is an autosomal dominant disorder with complete penetration and variable expressiveness characterized by the development of benign and malignant neural and extra neural tumours, “café au lait spots,, lesions in the viscera, skeleton, mental and cognitive disorders and adaptation difficulties. At the ocular level, NF1 is most frequently objectified by optical glioma and the presence of tumours on the conjunctiva, iris (Lisch nodules), choroid, retina. NF1 management is extended throughout the patients’ life and requires periodic clinical monitoring by neurologist, ophthalmologist, internist, simultaneously with CT scan, brain, spinal, abdominal, thoracic MRI to highlight tumours and to monitor tumour progression. NF1 cannot be cured but requires symptomatic treatment for the presence and spread of tumours and their complications, pain management, chemotherapy in optic gliomas, management of psychiatric disorders, surgical treatment indicated in extensive tumours (eyelid, brain, pheochromocytoma) and / or radiotherapy (limited for possible malignancy), laser surgery. Management of optic gliomas, although benign tumours, is difficult, symptomatic gliomas of the optic nerve with documented progression have indication for resection. Permanent clinical and imaging multidisciplinary monitoring is essential for a correct diagnosis, clinical course of the disease and appropriate treatment of NF1. NF1 is a polymorphic condition with variable, evolving, severe clinical features, sometimes life-threatening.
Keywords: Optic Glioma; Lisch Nodules (Iris Hamartoma); Neuroectodermal Dysembryoplasia; Hamartomas; Cutaneous Neurofibromas; Plexiform Neuroma; Bone Dysplasia; Intracranial Tumours and Spinal Cord; Isolated or Multifocal Meningiomas
Abbrevations: NF1: Neurofibromatosis Tip 1; Hamartooms: Tumors in NF1; NIH: National Health Institute; CNS: Central Nervous System; HTA: High Blood Pressure; NO: optic nerve; MRI: Magnetic Resonance Imaging; FDA: Food and Drug Administration; AHC: Antecedent Collateral Inheritance; BP: Blood Pressure; CT: Computed Tomography
Neurofibromatosis is the most common phacomatosis with an incidence of 1/3000 cases. Phacomatoses are hereditary and evolutionary neuroectodermal disembryoplasias with complete penetration and variable expression, produced by early embryogenesis disorders affecting the nervous system, eye and skin [1]. Clinically, there are hamartomas that are discrete at birth, but develop over time (years). Neurofibromatosis type 1 - NF1 - or von Recklinhausen’s disease is a polymorphic condition with variable clinical features, evolutionary, severe, sometimes life-threatening. NF1 is a neuroectodermal disembryoplasia in particular of the neural crest, which participates in the formation of the face and craniofacial skeleton, which gives rise to numerous histologically different migratory cells, which explains the lesion polymorphism of the disease. NF1 is a genetic, multisystemic disease with autosomal dominant transmission in which the mutation is located on the long arm of chromosome 17, position 17q112 [2]. NF1 can be produced by spontaneous or inherited mutation in the NF1 gene encoding neurofibromin, a protein present in neuronal cells. Neurofibromin inhibits the RAS pathway that regulates cell proliferation and differentiation [3,4]. In about half of the cases, the mutation occurs spontaneously (no family member has the disease), and the spontaneously acquired disease is passed on to future generations. Tumours in NF1 are hamartomas
i. In the CNS there are small-grade gliomas, clinically frequently asymptomatic.
ii. Intracranial spinal cord tumours.
iii. Cutaneous, subcutaneous neurofibromas, plexiform neuroma (malignant risk over time) located in the peripheral nervous system.
The diagnosis of NF1 is based on the presence of one or more of the following criteria:
i. 6 or more “café au lait spots” (> 0.5 cm in children, > 1.5 cm in adults)
ii. 2 or more cutaneous, subcutaneous or plexiform neurofibromas
iii. Freckles in the armpit and groin
iv. Optic glioma
v. 2 or more Lisch nodules (iris hamartom)
vi. Bone dysplasia - sphenoid wing, long bones with or without pseudoarthrosis
vii. The first case with NF1
Peripheral Nerve Damage:
i. Cutaneous neurofibromas> 99% - small round, subcutaneous, sessile or peduncled masses (sometimes multiple), localized or diffuse, in variable number, individually and familiarly.
ii. Plexiform neuromas, 30-50%, can cause significant morbidity because they are diffuse, grow along the nerve and infiltrate tissues [2], sometimes they can degenerate malignantly in the CNS
iii. 8-13% malignancies at the age of 20-35 years [3] that need to be diagnosed quickly and treated with surgical treatment, radiation therapy, adjuvant treatment or palliative treatment in patients with metastases and to reduce the size of the tumour for surgery.
i. Predilection for the optic nerve, brain with focal or diffuse tumours, occasionally aggressive: which are round, encapsulated tumours with slow growth, which do NOT degenerate, but may give compression pathology of cranial nerves or dorsal root of spinal nerves [4]
ii. Isolated or multifocal meningiomas that can invade the cortex and spinal cord [6].
i. Epilepsy 6-7% with cortical dysgenesis
ii. Cerebrovascular disorders [7]
i. “Café au lait spots” that do NOT give complications, but if they are unaesthetic the laser pigmentation may fade them (at the patient’s request)
i. Sympathetic, parasympathetic chain tumours
ii. Megacolon, megaureter
iii. Paragangliomas - pheochromocytoma, tumour of the carotid body
iv. Cardiovascular disorders with congenital heart disease, pulmonary artery stenosis
v. Pulmonary: Mediastinal mass with thoracic neurofibroma and / or pulmonary damage 20% with interstitial fibrosis, secondary hypertension
vi. Vascular: Aneurysm, arteriovenous malformations, renal artery stenosis, coarctation of the aorta.
Skeleton Damage: Kyphoscoliosis, neural foramen enlargement, dural ectasia, rib dysplasia, long bone pseudoarthrosis tibia, multiple fractures, fibromas [5]
i. Anxiety, depression, psychosis (respond to antidepressants)
ii. Sociopathic behaviour
i. Low intellect, learning difficulties without academic potential, sociocultural deficit
ii. Lack of socialization, hyperactive, autistic spectrum
iii. School difficulties with attention deficit
i. Plexiform Neuroma: may be the first skin sign that appears in NF1, at the age of 2-5 years with thickened, asymmetrical eyelid, which increases over time, with possible invasion into orbit.
i. Neurofibromas, plexiform neuroma, optic nerve glioma, optic nerve sheath meningioma [3]
ii. Optic tract gliomas are present at about 5 years old and are the second most common tumour in NF1 after neurofibroids [9]. Clinically, tumours are type 1 prechiasmatic retrobulbar glioma, type 2 optic tract glioma and type 3 chiasmatic glioma.
iii. NF1-associated gliomas generally have a good evolution, the unfavorable evolution factors being early onset (under the age of 6) and chiasmatic, retro chiasmatic localization
iv. Pulsatile exophthalmos due to the absence of the large wing of the sphenoid and intra orbital hernia of a possible encephalocele.
v. Bony arrangement of the orbital walls, damage to the large, small wing of the sphenoid, widening of the sphenoid and sphenomaxillary plate
Conjunctiva: Small prominent, bright spots (rare), which at biopsy are neurofibromas of the conjunctival nerves
Cornea: Gray lines that are hyperplastic corneal nerves
i. Iris - Lisch nodules - melanocytic iris hamartoma - small pigmented spots, brown / yellow, present in 80-90% above 6 years old [10].
ii. Glaucoma associated with / or NOT cataracts is possible
iii. Choroid
a. Neurofibroomas in the form of small yellowish, brown prominences
b. Uveal ectropion
c. Choroidal nevi
d. Choroidal hamartoma
e. Malignant choroidal melanoma is more common in NF1 than in the general population
iv. Retina
a. Hamartoma (rare)
b. Retinal astrocytoma
i. Optic nerve glioma and / or chiasm is the most common clinical manifestation in NF1> 15-30%
ii. May be present at birth
iii. Initially asymptomatic, over time is accompanied by decreased vision, nystagmus, optic atrophy or papillary edema
iv. Has a relatively slow evolution [9]
The positive diagnosis of NF1 is confirmed by the biopsy of neurofibroids which histopathologically confirms NF1 [5]. Complications in NF1 aggravate the course of the disease and are related to the location and spread of tumours. The prognosis of NF1 is variable without affecting life, in most cases is often uncertain, depending on the evolution of multiple lesions and their complications [2,7]. The most common causes of death in NF1 are cardiovascular complications or severe tumour progression.
NF1 management is extended throughout the patient’s (and sometimes family’s) life and requires: [4,11]
a. Patient and family education.
b. Specific, multidisciplinary clinical monitoring of the disease and the patient.
c. CT imaging control, brain, spinal, thoracic MRI, abdominal examination to highlight possible tumours.
d. Physiotherapy, kinetotherapy.
e. Symptomatic medical treatment:
i. Treatment with lovestatin would improve attention deficit and spatial orientation in children with NF1 [12].
ii. Rapamycin [13] would reduce the growth of astrocytes in vitro.
iii. FDA approves Koselugo (Selumetinib) the first targeted therapy for the treatment of children with NF1, with symptomatic, inoperable plexiform neuroma; It has been shown to reduce tumours and increase the quality of life in children [14].
iv. Cabozantinib - reduces tumour size and patient pain [14].
v. Chemotherapy - carboplatin, vincristine in optic glioma, vincristine, cisplatin in chiasmatic glioma [11].
vi. High doses of intravenous vitamin C, 7-15 g per week [15].
vii. Pain management.
viii. Radiation therapy is NOT indicated for the malignant potential and vascular, endocrine neuropsychic consequences.
ix. Surgical treatment in severe proptosis, glioma, optic chiasm.
x. Asymptomatic children from NF1 families should be ocularly monitored periodically for visual acuity (final at the age of 3), for chromatic sense (definitive at the age of 5), for visual field (definitive at the age of 8), correlated with MRI screening [4].
xi. High-risk patients should be regularly clinically monitored by multidisciplinary teams and by imaging techniques.
NF1 has a 50% familial genetic risk with definite morbidity and possible mortality from multiple and sometimes severe impairments. In the case of prenatal identification of the mutation, intrafamilial NF1 has a high clinical variability and it is difficult to recommend termination of pregnancy, because 50% of newborns will NOT have clinical expression, even if there are severe cases in the family.
The cases presented are a perfect mirror of the genetic transmission of NF1. The clinical manifestations of the patients followed personally over a long period of time (10-20 years) show a complex evolutionary pathology, extremely variable of the clinical determinations in NF1. The positive diagnosis of NF1 was established late in the presented cases (although in one of them there was the possibility of genetic transmission) based on clinical examination, heredocolateral antecedents in correlation with imaging exploration, CT, brain, orbital, spine MRI.
Patient C.S. currently 56 years old, without heredocolateral antecedents of NF1, is diagnosed with NF1 (probably by spontaneous mutation) at the age of 38 years after a neurological examination performed for epilepsy, with rarer seizures initially, starting the age of 30, epileptic seizures becoming more frequent in time. The patient is monitored by a multidisciplinary team from the age of 40 for NF1, the clinical evolution of the disease being complicated over time. Currently, the patient has a confirmed diagnosis of NF1 with “café au lait spots,, axillary freckles, skin neurofibromas, severe spastic ataxo tetraparesis, moderate / severe secondary dementia, epilepsy with grand mal seizures, organic personality disorder. Brain MRI shows benign left temporal brain lesion, hamartoma in the left brain. At ocular level, is diagnosed with corticonuclear cataract in both eyes, operated at the left eye in 2018 and primary open-angle glaucoma under treatment with cosopt and travatan. In NF1, the patient has a benign extended palpebral tumour in the inner corner and the edge of the eyelid which was excised in 2018. Particularity of the case:
i. Patient with NF1 probably by spontaneous mutation that transmits the disease to the first generation descendant.
ii. Benign tumours are small, slow-growing, unaccompanied by complications and sequelae.
iii. The eyelid tumour was detected early and operated in due time.
iv. Multidisciplinary monitoring of the patient; she has a child in whom NF1 manifests itself with tumours localized at cerebral and medullar level, which evolve and are accompanied by complications and serious sequelae, the evolution of NF1 being extremely severe in the child compared to the mother (Figure 1).
Patient C.C. - 36 years old at present, the son of the patient C.S. 56 years old with NF1, receives the diagnosis of NF1 at the age of 20, after a neurological examination for gait disorders that appeared with insidious onset and aggravated slowly and progressively. Positive diagnosis - NF1, spastic tetraparesis, C1-C2 spinal cord compression, left leg deformities (equine varus).
i. Neurological examination at the age of 20: good general condition, multiple “café au lait spots,, of variable size, 1 cm, located in the leg, abdomen, chest, right armpit, at lumbosacral level, generalized skin tumours, painless, mobile in size and varied consistency, left leg deformed in equine varus, no signs of meningeal irritation, mowing, spastic gait, symmetrical, reactive pupils, spastic tetraparesis with predominantly left hemiparesis, preserved sphincter control.
ii. Brain MRI at the time of diagnosis of NF.
iii. Without focal brain lesions, extradural cervical neurofibromas with C1-C2 medullary compression, cutaneous, epicranial neurofibromatosis.
iv. Lumbar dorso-lumbar spine MRI - C2-C5 prolapse with C2 medullary imprint without root compression. Multiple C-D-L-S intraforaminal formations with root compressions, medullary C1 - neurofibromatosis appearance.
v. Neurosurgical examination - C1-NF1 spinal cord compression.
vi. Chest CT examination 2018 - multiple skin and subcutaneous thoraco abdominal nodules, several right subpleural nodules.
vii. MRI 2021 - benign tumour of peripheral nerves and CNS, myelopathy, NF1 (non-malignant), spastic tetraparesis, high cervical compression syndrome, multiple cervical neurinomas C2-C3, NF1, CSF drainage device.
viii. Native skull CT - 2022 - post-shunt appearance, left macronodular foraminal lesion C3-C4 with medullary compression, diffuse epicranial subcutaneous fibromas.
Histopathological examination 2015 on 3 pieces collected from the left, right intradural cutaneous neurofriboma - confirms the diagnosis of neurofibroma in the 3 pieces. The patient is currently provided with:
i. Dispensarisation by the family doctor
ii. Periodic multidisciplinary monitoring
iii. Psycho hygiene measures
iv. Kineto therapy, physiotherapy, medical gymnastics
v. Psycho protective family climate
vi. Psychosocial support
vii. Has full assistance in carrying out all daily activities
viii. Supportive medical treatment
i. Patient with NF1 with autosomal dominant genetic transmission with severe evolution.
ii. The diagnosis of NF1 was established late (at the age of 20), due to the non-addressability to the medical services, although the patient had heredocolateral antecedents and clinical symptoms.
iii. The clinical manifestation of NF1 is in the form of cerebral and spinal hamartomas that gave compression and were followed by complications and severe sequelae with the current immobilization of the young patient in bed.
iv. The clinical evolution was relatively fast and dramatic, although this patient was regularly monitored correctly (Figure 2).
Figure 2: There are multiple schwannomatous masses at the cervical conjugation holes, the most voluminous being located at C2-C3 left, with a diameter of 35mm and with multidirectional evolution, both in the root canal and in the spinal canal, where it compresses the medullary cord and displaces it to the right, without hydro or syringomyelia.
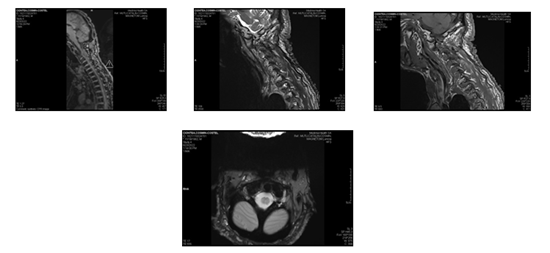
Patient I.P.E. 21 years old is currently diagnosed with NF1 at the age of 17, following pediatric neurological evaluation for headache syndrome.
i. Febrile seizures at 3 years, sinusitis at 14 years, left ankle fracture (possible minor post-trauma).
ii. Physiological puberty
Objective Examination: Waist 156 / weight 51, multiple “café au lait spots,, scattered on the trunk, abdomen, limbs, dimensions a few mm to 5cm, freckles at axillar and groin level, harmonious skeleton, normal tibia, normal muscles, blood pressure 125/89, AV 78 / min rhythmic, inconsistent frontal headache, painful sinus points.
Neurological Examination: 2018 – deep tendon reflexes present, symmetrical, slight palpebral ptosis left> right, normal cranial nerves, appropriate language, excitability, immaturity.
MRI with contrast substance: 2018 Intraorbital right optic nerve slightly increased, 6 mm in size, cross section on a length of 17 mm from 8 mm from the eyeball to the level of the upper orbital fissure – optic nerve glioma. Multiple left cerebral hamartomas and at thalamus level, benign intercranial hypertension.
Clinical Examination 2020: Growing “café au lait spots,, axillary freckles, thyroiditis, hereditary and idiopathic neuropathy.
Native Brain MRI - 2020 - compared to 2018 - intra and supraterritorial hamartoma lesions described in 2018, appear numerically reduced. The remaining lesions in the corpus callosum appear with dimensional and stationary regression at the level of splenic corpus callosum. Stationary appearance of right optic nerve glioma. The patient is currently monitored for NF1 with right optic nerve glioma with periodic clinical, imaging control. The peculiarity of the case
i. Timely diagnosis of NF1, the patient presenting small tumours.
ii. Brain and optic nerve tumours should be monitored periodically clinically and by imaging techniques.
iii. NF1 is probably produced by spontaneous mutation.
iv. The patient has a small child (1 year old) who must be investigated as soon as possible (Figure 3).
About half of NF1 patients inherit the disease from the affected parents, if the NF1 patient has no affected relatives, it probably has a new genetic mutation. NF1 has an autosomal dominant transmission, so any child with an affected parent has a 50% risk of inheriting the genetic mutation. Symptoms and signs in NF1 are present in childhood, adolescence, are evolutionary and can be accompanied by multiple complications:
i. Neurological - epilepsy, hydrocephalus, learning difficulties.
ii. Visual disturbances in optic glioma.
iii. Skeletal Disorders: kyphoscoliosis, decreased bone mineral density at risk of osteoporosis, kyphoscoliosis.
iv. Respiratory disorders in plexiform neurofibroma (rare).
v. Cardiovascular disorders: With an increased risk of hypertension and vascular abnormalities.
vi. Malignant tumours in 3-5% of patients with NF1 by malignancy of neurofibromas or plexiform neuroma.
vii. Pheochromocytoma benign tumour of the adrenal gland - produces paroxysmal hypertension and requires surgical excision.
Patient monitoring begins with a careful history of the patient and family, and a multidisciplinary clinical examination tailored to the disease and the patient, correlated with:
i. Imaging Tests - CT, MRI used to diagnose and monitor the evolution of NF1.
ii. Genetic tests.
iii. Histopathological examination.
Child monitoring recommends adequate annual checks with: skin examination, assessment of the child’s growth and development (height, weight, head circumference), signs of early puberty, skeletal abnormalities, school progress. Adults with NF1 require periodic evaluation with: careful examination of the skin for neurofibromas, bone evaluation, BP measurement, neuropsychiatric evaluation. NF1 cannot be cured, but symptomatic treatment is required for the presence and spread of tumours, their complications, pain management, chemotherapy in optic gliomas, management of psychiatric disorders, surgical and/or radiotherapy, laser surgery or electrocautery. If the neurofibroids are small, treatment is NOT necessary; In large tumours, surgical treatment and plastic surgery are required. Surgical treatment of plexiform neurofibromas is difficult due to the spread of the tumour, and postoperatively, nerve damage is possible.
NF1 is a multisystem genetic condition with autosomal dominant transmission, which has variable clinical aspects and has a serious evolution, sometimes life-threatening. The most common clinical manifestations are given by the presence of cutaneous and subcutaneous tumours, neurofibroma and plexiform neuroma. The ocular manifestations of NF1 are multiple, with the frequent presence of optic glioma (optic nerve, optic chiasm), Lisch nodules. NF1 management is extended throughout the patient’s life, high-risk patients should be regularly clinically monitored by multidisciplinary teams and by imaging techniques. In NF1 there is definite morbidity due to multiple and sometimes severe clinical impairment, which must be properly diagnosed and treated. The treatment is medical, symptomatic, radiotherapeutic, chemotherapeutic and surgical.
The authors did not report any potential conflicts of interest in research, authorship, and / or publication of this article.
The authors have not received any financial support for the research, authorship and / or publication of this article.


