Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Shashikumar K Paknikar1* and Nagaraj Gouda2
Received: June 30, 2022; Published: July 12, 2022
*Corresponding author: Shashikumar K. Paknikar, Siddharth Chemicals, Kundai Industrial Estate, Kundai, 403115, Goa, India
DOI: 10.26717/BJSTR.2022.45.007147
Uguenenazole 6, has been synthesized in by refluxing a mixture of 4-methoxy phenacyl bromide 9 and benzamide 10 in chlorobenzene. It is proposed that initially formed uguenenamide 7 undergoes an acid catalyzed cyclization in situ to produce 6. The reported isolation of 6 and 7 from Vepris ugenensis Engl (Rutaceae) clearly shows that the biogenesis of 6 involves an intramolecular acid catalyzed cyclization of 7.
Keywords: Synthesis of Uguenenazole; 2,5-Diaryl-1,3-Oxazole; Vepris Ugenensis Engl; 5-(4-Methoxyphenyl)-2-Phenyl Oxazole
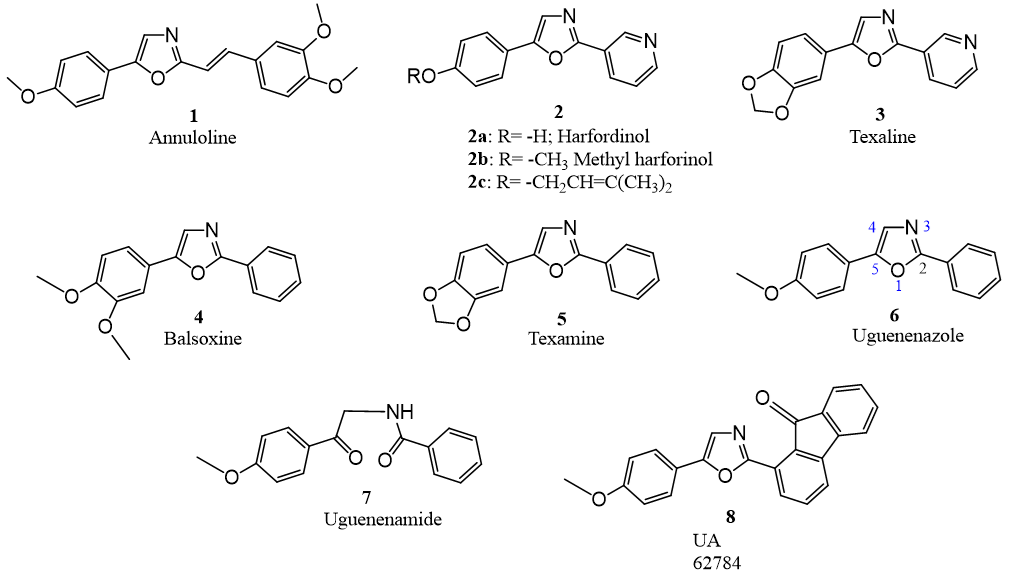
The plant family Rutaceae is known to be a rich source of coumarins and alkaloids. Oxazole alkaloids with aryl substitution both at 2 and 5 positions isolated from few genera of Rutaceae family received a considerable attention from natural product chemists and synthetic organic chemists due to their simple structural features and amazing biological activities. Although several 2,5-diaryl substituted oxazoles have been isolated and characterized from marine sources, we limit ourselves with only a small group of naturally occurring compounds isolated from diverse plants belonging Rutaceae. Incidentally, annuloline 1, the first natural oxazole isolated and characterized in 1962 occurs in seedlings of the annual rye grass (Lolium multijorum) [1] while the other oxazole alkaloids, halfordinol and its derivatives 2a, 2b and 2c [2-4], texalin 3 [5], balsoxin 4 [6], texamine 5 [7], uguenenazole 6 and its precursor ugenenamide 7 [8] have been reported in plants belonging to family Rutaceae (Figure 1). Uguenenazole 6 and its precursor ugenenamide 7 happen to be the recently reported natural products from Rutaceae. This is the first report of isolation of a precursor and oxazole together from the same source.
Much before the isolation and characterization of 2,5-diaryl-1,3- oxazoles from rutaceous plants, a method for the synthesis of 2,5-diaryl oxazoles was reported in view of their use as scintillators in medical diagnostics [9]. The natural plant oxazoles were found to exhibit various biological activities and due to this, oxazoles gained importance from synthetic organic chemists and those involved in medicinal chemistry. Moreover, it is observed that the more complex natural products which contain oxazole moiety exhibit several biological activities [10] such as cytotoxic [11], antifungal [12], antibacterial [5], antitumor [13] and antiviral activities. In view of many research articles reporting the synthetic methodologies for 2,5-diaryloxazoles, we have referred only those which are directly related to the naturally occurring 2,5-diaryl substituted oxazoles 1 to 6 (Figure 1) and those which report their synthesis.
Figure 1: Naturally occurring 2,5-diaryl oxazoles and an amide from plant sources except 8 (synthetic).
Scheme 1: One-pot synthesis of uguenenazole 6. Reagents and conditions:
1. Reflux in chlorobenzene.
2. HBr (generated in situ).
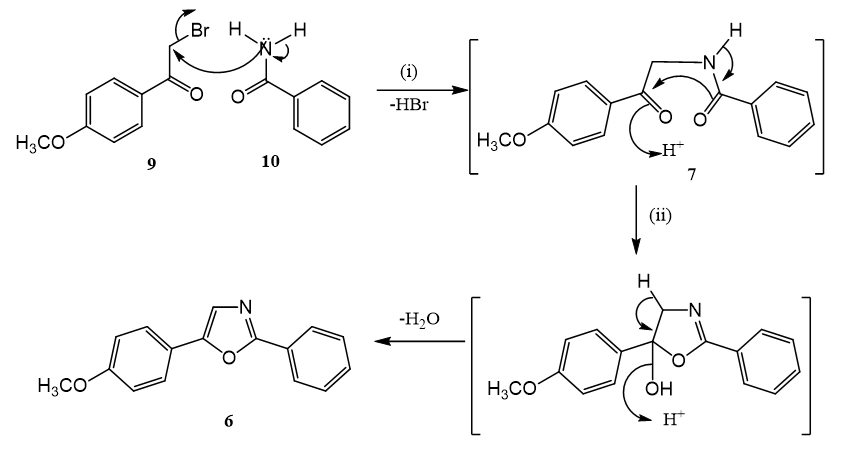
A simple one pot synthesis of uguenenazole 6 is reported herein (Scheme 1). By refluxing commercially available 2-bromo- 4′-hydroxyacetophenone 9 and benzamide 10, in chlorobenzene for 1 hr. uguenenazole 6 was obtained in 59% yield. Benzoic acid was also isolated in a small quantity. In order to explain the formation of 6 under the conditions used, we believe that the in situ generated uguenenamide intermediate 7 undergoes an intramolecular acid catalyzed cyclization where HBr generated acts as a catalyst. Formation of benzoic acid is a result of acidic hydrolysis of benzamide 10 and/or of the intermediate uguenenamide 7. Comparison of the spectral data measured on synthetic uguenenazole with those recorded on the natural product established their identity beyond doubt. It may be noted that uguenenazole 6 has been synthesised twice before [14,15] as an application of newly developed methodologies for 2,5-diaryl-1,3-oxazoles.
Uguenenazole 6 and uguenenamide 7 have been isolated from the roots of Vepris ugenensis Engl. (Rutaceae). A plausible biosynthetic link has been proposed [8] and 6 and 7 have product– precursor relationship. We have now demonstrated that the key step of the proposed pathway can be achieved using a simple single step process. A new question is to be answered what if ugenenamide 7 is the product then what is its precursor? A search and experimentation in this direction would answer this question. Cheplogi et al. [8] have reported EIMS of natural uguenenazole 6. We studied the mechanism of origin of the major fragment ions and the same is presented in Figure 2. 2,5-Diaryl-1,3-oxazoles have a great potential for the development of lead compounds in the search for the new drugs and medicines. One such molecule 8 UA62784 (Figure 1) where 5-(4-methoxyphenyl)- substituent of uguenenazole 6 is retained and the substituent at 2 position is modified for the synthesis of a new lead molecule UA 62784 8 (Figure 1) and being studied as an antitumor agent for the treatment of pancreatic cancer [13].
One pot synthesis of uguenenazole: In 25 mL R B Flask equipped with a magnetic stirrer, reflux condenser, 2g of p-methoxyphenacyl bromide(1eq) and 1.06g of benzamide(1eq), then added 4.9g of Chlorobenzene (5eq), the reaction mass was refluxed for 1.5hr, the reaction was monitored by TLC control system (Hexane: Ethyl aceatae-7:3). TLC shows a fluorescent spot on non-polar region. The reaction mass was concentrated under reduced pressure, then the residue was treated with 10 mL water, then extracted with 20mL ethyl acetate, the organic layer was washed with 10 mL water, then dried over Na2SO4, then filtered off, concentrated. The crude product was purified by column chromatography using hexane–ethyl acetate system. (4%ethyl acetate in hexane). The pure white product was isolated (1.3g, 59%, unoptimized). Melting point: 126-128oC. Mass analysis: 269.29(M+18) and 274.2 (M+ Na). 1H NMR (400 MHz) (CDCl3) δ 8.12 (2H, dd, J=8.0,1.8 Hz), 7.88(1H, s), 7.50-7.40 (3H, m), 7.76(2H, d, J=9.0 Hz), 6.97 (2H, d, J=9.0 Hz), 3.86 (3H,s); 13C NMR (100 MHz) (CDCl3):δ 161.60, 159.59, 141.7, 128.7, 127.5, 126.9, 126.45, 123.84, 114.17, 55.36.
In conclusion, uguenenazole 6 was synthesized using 4-methoxyphenacyl bromide 9 and benzamide 10 in one-pot reaction protocol. The two-component reaction mixture was refluxed using chlorobenzene as solvent. This operationally friendly and simple procedure has excellent scope for synthesis of other naturally occurring 2,5-diaryl-1,3-oxazoles and those having potential uses in synthetic and medicinal chemistry.
We are grateful to Dr. Dnyaneshwar Gavas, (Institute of Organic Chemistry, Saarland University, Germany) for 1H and 13-CNMR spectra on our synthetic Uguenenazole and Professor Shailesh R. Shah (The Maharaja Sayajirao University of Baroda/Indrashil University, Rajpur) for helping in preparation of this manuscript.
**This paper is dedicated in the loving memory to Professor Robert B. Bates, University of Arizona, Tucson, Arizona, USA who passed away on June 11,2020
*Thermal reaction between 4-methoxyphenacyl bromide and benzamide.
*In situ generation of uguenenamide, the precursor of uguenenazole.
*One pot synthesis of uguenenazole by intramolecular acid catalyzed cyclization.


