Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Attah Caleb Joseph1*, Daniel Zanyu Egah2, Adgidzi Godwin Adgidzi1, Nandi Ishaya Tokkit3, Paul Alumbugu Tsaku4 and Alabi Oluwapelumi Oyinkansola5*
Received: June 25, 2022; Published: July 12, 2022
*Corresponding author: Attah Caleb Joseph, Department of Paediatrics, FMC Keffi, Nigeria
Alabi Oluwapelumi Oyinkansola, Department of Medical Microbiology and Parasitology, University of Ibadan, Nigeria
DOI: 10.26717/BJSTR.2022.45.007145
The global target set by the World Health Organization is to reduce malaria incidence and deaths by 90% by 2030. Rigorous efforts have substantially decreased the malaria burden over the years, but it still remains a threat to lives of millions of children especially in the tropics. The need for development of an effective malaria vaccine has become imperative and reasonable efforts have been channelled towards the discovery of a successful malaria vaccine. The malaria parasite, unlike most bacteria and viruses, has a complex biology coupled with its intricate infection cycle that makes it more difficult to develop an effective malaria vaccine. This review discusses the different approaches to malaria vaccine development as well as the progress and prospects of malaria vaccine candidates with emphasis on RTS, S/ASO1 vaccine.
Keywords: Malaria; RTS, S/ASO1 Vaccine; Plasmodium
Malaria has remained a leading cause of morbidity and mortality in the 21st Century especially in Sub- Saharan Africa in spite of all intervention efforts aimed at eradicating the disease [1,2]. Globally, an estimated 405,000 deaths out of 219 million clinical cases of malaria were reported in the year 2018; where children ≤5 years accounted for 67% of these figures, out of which 265,000 were African children [3,4]. These massive numbers nonetheless indicate that remarkable progress has been made especially in the last two decades; the total estimated deaths from malaria was about 1 million in 2000, and 780,000 in 2009 [5]. The reduction in malaria morbidity and mortality in recent times is a result of the scaling- up of malaria control measures by the global health community. Such scale-up measures include the use of long-lasting insecticidal nets (LLIN), indoor residual spraying programmes (IRS) and access to artemisinin combination therapy (ACT) [6]. Additionally, the use of rapid diagnostic tests instead of presumptive treatment of malaria has significantly contributed to the gains achieved in the control of malaria [5].
A major setback to the target of eradicating malaria in the nearest future however is the emergence of resistance to existing antimalarial drugs and insecticides, and the possible presence of asymptomatic and submicroscopic infections [5]. These necessitated the need for vaccines to prevent the continuing spread of malaria. The development of vaccine against malaria began more than fifty years ago [7], but due to the extreme complexity of malaria parasites’ biology, complex and diverse parasites’ genomes, and immune evasion by the parasites as well as the intricate nature of the parasite’s infection cycle [8,9], a fully licenced vaccine has not been achieved yet. The Plasmodium life cycle is in three stages and undergoes both asexual and sexual reproduction within two different hosts. The first two stages, namely, pre-erythrocytic and erythrocytic stages, involve asexual reproduction within the human host, while the third stage involves sexual reproduction within the mosquito gut. This makes it a huge challenge for researchers to design an ideal malaria vaccine [3].
To date, only the RTS, S/ASO1 vaccine candidate has been approved for pilot implementation in 3 to 5 Sub-Saharan Africa settings. The term ‘malaria’ first appeared in English medical literature in 1829 [3]. It originated from the Italian ‘mal’(bad) and ‘aria’(air), when it was initially thought to be caused by foulsmelling air near swamps long before Louis Pasteur postulated the Germ Theory of Diseases. Malaria is caused by five protozoan species, namely Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi [6]. Over 90% of malaria-related deaths are caused by P. falciparum which is predominant in sub-Saharan Africa [5,6]. Equally, over 90% of malaria vaccine development projects are dedicated towards elimination of P. falciparum malaria.
Understanding the immune responses to malaria parasites is crucial for the development of effective vaccines. The possibility of achieving immunity against malaria is backed by a number of evidence. The demonstration by Cohen, et al. [10] that partial immunity to malaria could be achieved in children treated with purified gamma-globulin fractions from semi-immune adults is a major pointer towards the goal of malaria vaccine development. Similarly, Clyde, et al. and Cochrane, et al demonstrated that inoculation of humans with irradiated sporozoites by mosquito bite can prevent the emergence of blood-stage infection [11,12]. In endemic areas with natural exposure, sterile immunity rarely if ever develops. Perhaps most importantly and significantly, the candidate vaccine RTS, S/ AS01 can induce clinical efficacy in the 25- 60% range in different malaria endemic settings. Thus, the question of feasibility of malaria vaccination has progressed to an assessment of the public health role of RTS, S vaccination and the possibility of developing even more efficacious second-generation vaccines [5].
Basically, Malaria vaccines are designed to target one of the three stages in the life cycle of Plasmodium species.
The pre-erythrocytic stage is the period where sporozoites of Plasmodium injected into the human subcutaneous by a bite of an infected Anopheles mosquito travel through blood to infect hepatocytes and undergo schizogony, the vigorous multiplication stage that precedes the invasion of red blood cells (RBCs). The aim of developing a vaccine against this stage is to impede hepatocyte infections thereby preventing parasitic invasion of the red blood cells [13,14]. A completely effective pre- erythrocytic vaccine would inactivate the parasite before it leaves the liver, leading to sterile immunity and prevention of disease [5]. The mechanisms of protection for this stage may involve antibody responses that prevent sporozoites from invading hepatocytes or cytotoxic T cells that destroy infected liver cells [6]. As shown in the Table 1 below, there are currently 12 pre-erythrocytic vaccine candidates under development for plasmodium falciparum, of which the RTS,S malaria vaccine is the most advanced [3].
Table 1: Malaria vaccine projects targeting the pre-erythrocytic stage.
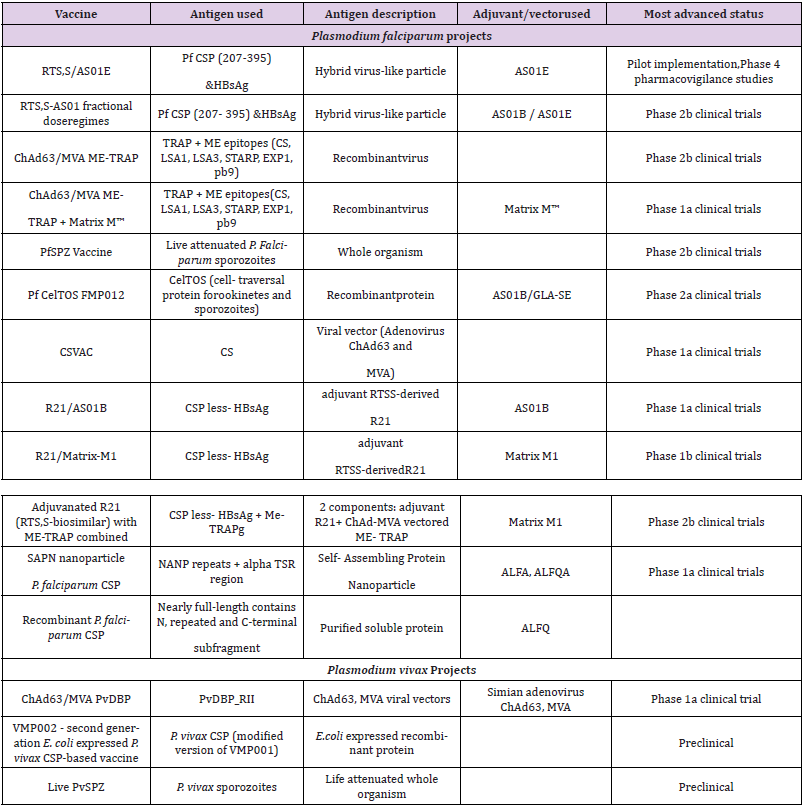
Note: Data source: WHO [2017]
Subsequent to the pre-erythrocytic stage in Plasmodium life cycle is the erythrocytic stage where merozoites are released from the liver into the bloodstream to infect erythrocytes. Vaccines designed to target this stage are aimed at preventing the invasion of the RBCs by malaria parasites. The motivation for developing such vaccine candidates comes from evidence that people with recurrent malaria infections in endemic areas develop some level of protective immunity, a state in which there is immunecontrolled RBC invasion, resulting in fewer disease symptoms or asymptomatic infections [10,15]. The mechanism of action of erythrocytic vaccines is mediated through antibodies that target the merozoite surface proteins (MSP), such as MSP-1, thereby halting invasion of RBCs [2]. Other blood- stage vaccines target parasite antigens embedded in infected RBC membranes, such as P. falciparum Erythrocyte Membrane Protein-1 (PfEMP1) [16]. There are currently 15 erythrocytic or blood stage vaccine candidates under development [3] (Table 2).
Table 2: Malaria vaccine candidates targeting the blood stage of malaria infection.
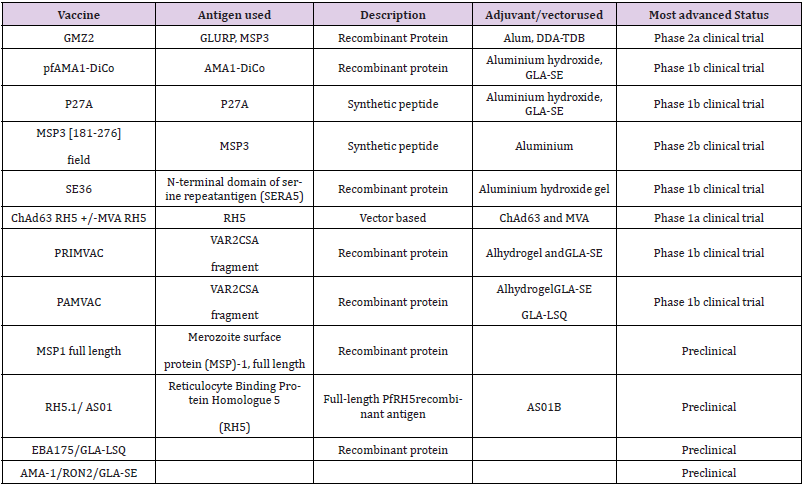
Note: Data source: WHO [2017]
This vaccine candidate target is the sexual parasite forms or gametocytes. Proof of concept was first reported in the 1970s, but substantial progress and political traction has only happened over the last decade, in line with renewed calls for malaria elimination [1]. At the end of the erythrocytic stage, a proportion of the merozoites differentiate into sexual stages, which are taken- up by an Anopheles mosquito when it bites an infected person. The parasite completes its life cycle within the gut of the mosquito. The transmission blocking vaccines target the mosquito gut to stop sexual reproduction of the parasite. They are so called because they aim to kill the mosquito to block further transmission of the parasite. These vaccines generate antibodies that either prevent fertilization of the gametes in the mosquito gut or stop the development of the zygote into sporozoites. They do not confer direct protection to the immunized individual but produce herd immunity. Table 3 below represents the transmission blocking vaccine candidates currently under development [3].
Table 3: Malaria vaccine projects targeting sexual stage of the parasite.
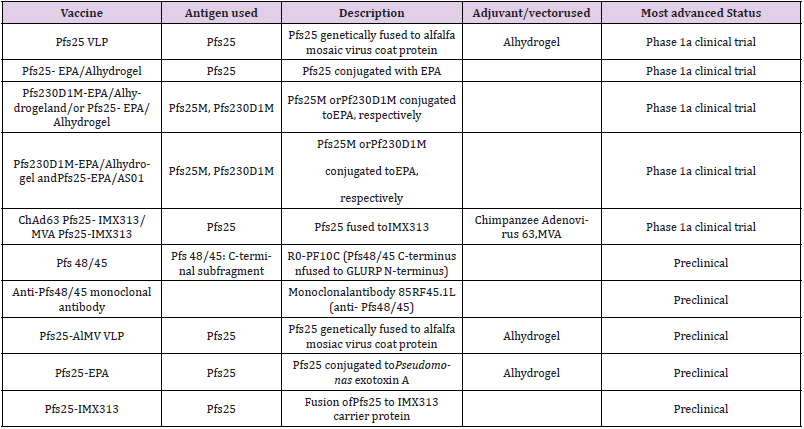
Note: Data source: WHO [2017]
There are over 30 malaria vaccine candidates that are undergoing clinical trials or in advanced preclinical development. The RTS, S/AS01E is by far the most advanced malaria vaccine. Some major malaria vaccine candidates are highlighted in Figure 1. More emphasis is given to the RTS, S/AS01E vaccine candidate here due to the promise it has shown in all its developmental stages.
The most advanced malaria vaccine, which has passed all clinical trials and has been approved as the world’s first malaria vaccine is RTS, S/AS01E (for pediatric use), also known as Mosquirix TM [3]. It is a recombinant vaccine, consisting of virus-like particles (VLPs) made by expression of the hepatitis B surface (S) antigen (HBsAg) in the yeast Saccharomyces cerevisiae. The S antigen is fused to the circum sporozoite protein (CSP) of P. falciparum, containing the repeat region (R) and a T-cell epitope (T). The vaccine is formulated with AS01, which is a liposome-based vaccine adjuvant that boosts the immunogenicity of the vaccine. The RTS, S malaria vaccine development began in 1984 at the Walter Reed Army Institute of Research (WRAIR), Silver Springs, Maryland, USA. Over the next 30 years, the vaccine was taken forward by GlaxoSmithKline (GSK) with collaboration from PATH’s Malaria Vaccine Initiative (MVI) [17]. The Phase 3 clinical trial was conducted in 7 African countries over a span of 5 years (2009-2014) by GSK and MVI, with funding from the Bill & Melinda Gates Foundation (BMGF). This Phase 3 clinical trial included a total of 15,460 participants, including 6,537 infants and 8,923 children, aged 5-17 months.
These infants and children were vaccinated either 3-times or 4-times with the RTS, S malaria vaccine or a control vaccine (meningococcal C vaccine for infants and rabies vaccine for children). Vaccine efficacy was based on the reduction of the number of clinical malaria cases, severe malaria cases, and malaria hospitalizations. The immunogenicity of the vaccine was determined by its antibody inductive capacity against CSP. The impact of the vaccine on disease burden was estimated on the basis of the number of clinical and severe malaria cases averted per 1,000 immunized children. The follow-up period, post- vaccination, was 4 years. After 4 years of follow-up, it was found that the protection conferred by the RTS, S malaria vaccine against clinical malaria was 36.3% (95% CI: 31.8- 40.5) among children 5-17 months of age who had received all 4 doses of the vaccine. In the case of children who received 3 doses of the vaccine, protection against clinical malaria was 28.3% (95% CI: 23.3-32.9). On the other hand, young infants who were aged 6-12 weeks at the time of first vaccination recorded a significantly lower protection against clinical malaria at 25.9% (95% CI: 19.9-31.5) in those who received 4 doses and 18.3% (95% CI: 11.7-24.4) in those who received 3 doses. Therefore, protection was lower in the 6–12- week group, compared to the 5-17 months group [18].
The RTS, S vaccine also provided significant protection against severe malaria and substantially reduced the number of hospital admissions arising from malaria in case of children who received all 4 doses. In case of children who received all 4 doses, approximately 1,774 (95% CI: 1,387-2,186) clinical malaria cases were averted per 1,000 vaccinated children [19]. However, two negative aspects of the RTS, S vaccine were noticed in this clinical trial. The first is that the vaccine is associated with an increased risk of febrile seizures within 7 days of the administration. However, the children who experienced these febrile seizures recovered completely with no lasting consequence. The second was the waning of the vaccine efficacy over time, resulting in rebound malaria cases, also termed as “age shift” [3]. While this raises some concern, other studies have shown that this is not unique to vaccine use. Other interventional methods such as the use of long- lasting insecticide-treated nets, mass drug administration and chemotherapy and prophylaxis strategies, also record some degree of age shift phenomenon [20,21].
The RTS, S vaccine is the only approved malaria vaccine currently undergoing pilot implementation in three African countries, namely Malawi, Ghana and Kenya. The pilot implementation which was launched in 2019 is expected to be completed by 2023. In a statement released on the 25th of April in celebration of the 2022 World Malaria Day, WHO announced that more than one million children had received the malaria vaccine. The Director-General of WHO, Dr Tedros Adhanom Ghebreyesus also stated that the RTS, S vaccine is a breakthrough for science, child health and malaria control; a game changer that is arriving at the right time. Furthermore, the former WHO Director of the Global Malaria Program, Dr Pedro Alonso also reckons “What we have right now is a vaccine that can be deployed, that is accepted, that is safe, and that can have a massive impact.” The World Health Organization recommends that the use of the RTS, S/AS01E vaccine be employed in the prevention and control of malaria caused by plasmodium falciparum, particularly in children living in regions with moderate to high transmission as defined by WHO. WHO also recommends that the RTS, S vaccine be given in a schedule of four doses in children from five months of age [22-32].
As proposed by the World Health Organization, the RTS, S/ ASO1 vaccine currently undergoing pilot implementation in Ghana, Kenya, and Malawi which started in 2019 is hoped to excel based on the early findings from the first two years data and insights gathering of vaccination activities in child health clinics in the three pilot countries [32]. Some of the findings include the RTS, S/ASO1 vaccine having a high delivery feasibility through the use of routine immunization systems as confirmed in the pilot countries, even during the COVID pandemic. Also, the use of routine immunization systems in the vaccine delivery enables reaching the unreached, particularly children with limited access to bed-nets or other preventive intervention [22,32]. With a significant 30% reduction in death rates from severe malaria as recorded in the pilot implementation, the vaccine has undoubtedly a reasonable impact in real-life vaccination settings, having a strong and favorable safety profile, while also being cost-effective. Although the RTS, S malaria vaccine introduction did not have an impact on the uptake of other routine childhood vaccinations, ITN use, health care seeking behaviors for febrile illness, or other child health interventions in the pilot implementation, it may be too early to conclude that it would not impact them in the nearest future with further modifications [32-38].
The RTS, S vaccine may still contribute to strengthening health interventions and increasing uptake of other vaccines in the near future. With these aforementioned key findings and successes recorded, it is hoped that these results from the programs will enable policy makers to provide an update on malaria vaccine policy and make recommendations for the wider use of RTS, S/ ASO1 for routine immunization [38,39]. For example, the pattern of adopting routine immunization in the three pilot countries can be replicated in other Sub Saharan African countries like Nigeria where their National Programme on Immunization (NPI) can allow immunization of a five-month infant to take the three primary doses of the vaccine within eight weeks with a four week spacing interval between the first, second and third doses, while the fourth dose may be taken at 15 months to coincide with the second dose of vitamin A on the Nigerian NPI schedule. This will warrant minimal adjustment on the routine immunizations already in place. Additionally, the consideration of a fifth dose in areas with highly seasonal malaria or with perennial malaria transmission with seasonal peaks as recommended by the WHO, can be structured and administered alongside other vaccines on the supplementary immunization days which comes annually. Table 4 shows the proposed adjustment for Nigeria’s NPI schedule to include RTS, S/ ASO1 vaccine.
The goal of these adjustments is to enhance ease of administration, efficiency and increase vaccine uptake among the targeted groups, which is cost effective [39]. Furthermore, malaria is a disease familiar to most parents, especially in Sub-Saharan Africa. An introduction of the vaccine is less likely to receive resistance as it addresses a problem that all can see and relate to. A particular study done in Nigeria showed that 76% of caregivers would be willing to allow their children participate in malaria vaccine test trials and 98% stated they would appreciate it if the vaccine became a more accessible reality [40]. This suggests that parents, particularly mothers, would appreciate the vaccine better and may eventually become advocates, leading to wider acceptance of the vaccine. The WHO recommends that the RTS, S/ASO1 malaria vaccine be provided as part of a comprehensive malaria control strategy as virtually all malaria control interventions may be only able to provide partial protection and thus the highest impact could only be achieved when multiple interventions were used concomitantly [32,38,39]. It is thus the responsibility of National Malaria Control Programs to identify and employ interventions which best suit them taking cognizance of their local malaria epidemiology such as malaria transmission intensity, age at which severe malaria is most prevalent, vector species and insecticide resistance pattern. Contextual factors which apply to their peculiar settings such as structure, function and organization of their health care system must also be considered in determining choice of malaria control interventions [41,42].
The WHO also notes that the additional visits needed to have a child immunised with the RTS, S/ASO1 vaccine provides opportunities for other integrated and preventive health services [41]. These visits may be used to catch up on missed vaccinations, administer vitamin A and carry out deworming exercises among other preventive health interventions [41-43]. These visits may also serve to educate and remind parents of the importance of other ancillary malaria control and health promoting interventions such as the use of insecticide treated nets and to seek prompt diagnosis and treatment of common childhood ailments especially fever and convulsions [45]. These visit sessions would also prove very valuable as a platform for ongoing surveillance and the gathering of information about the newly introduced immunizations and the whole immunization process in general [46]. While the vaccine records a significantly high efficacy in children, similar studies will need to be conducted on adults and pregnant women who are also significant carriers and reservoirs of malaria in malaria-endemic regions [41, 43-46].
Although the RTS, S/AS01 vaccine is the first to get to phase 3 in the pipeline and subsequently be launched, other vaccines being developed in the pipeline in Figure 1 show promising results. The ChAd63/MVA ME-TRAP and pfs PZ vaccines for instance, which are also pre-erythrocytic stage vaccines are currently in the phase 2b of the pipeline, coming just behind the recently launched RTS, S/AS01 vaccine [47]. In addition to the pre-erythrocytic stage vaccines being developed, vaccines in other stages of the parasite lifes cycle such as the erythrocytic and sexual stages are also being explored with encouraging progress [48]. AMA1-DiCo and P27A are both erythrocytic stage malaria vaccine candidates that have progressed to the phase 1b clinical phase trials. Pfs25-EPA, another malaria vaccine candidate in the transmission-blocking or sexual stage has also progressed to phase 1b clinical phase trials [47,48]. With encouraging results being recorded, research on these vaccines in development should not be neglected or halted, instead, more resources should be invested in them. Beyond the three major approaches discussed earlier; pre-erythrocytic, erythrocytic, and sexual stages, other approaches to vaccine development are also being explored by scientists. The possibility of multistage/multiantigen and whole organism control vaccines are being researched with hopes that through further modification and genetic engineering, can produce results that will surpass the results of the RTS, S/AS01 vaccine [49]. Furthermore, most of the vaccines currently being developed in the pipeline, including RTS, S/AS01 are designed to prevent malaria caused by plasmodium falciparum species [47-49]. This is expected as P. falciparum is responsible for the highest burden of malaria. However, other plasmodium species such as P. vivax, P. ovale, and P. malariae that have a low incidence rate still have significant impact on the burden of malaria globally, and more vaccines are being explored to tackle their impact [50].
The longstanding obstacle in malaria vaccine development which poses a challenge has remained the absence of immune correlates of protection for malaria vaccine [51]. The discovery of a biomarker which could behave as a reliable proxy of protection against clinical diseases, together with the availability of a predictive animal model (currently existing, but suboptimal) would make development efforts much easier and more efficient [51,52]. Many knowledge gaps need to be filled regarding naturally acquired immunity, its molecular and epidemiological determinants [48,51]. The complexity of the parasite as compared to viruses or even bacteria is clearly another limiting factor [8]. Any of the malaria plasmodium species presents thousands of antigens, which differ depending on the parasite stage of the cycle in both the human host and vector [53]. Moreover, the immune responses against different stages of the parasite have been proven to vary, hindering the possibility of finding those who play a major role in triggering human immunity, which would be the desirable candidates for malaria vaccine [8,48,49,51,]. In addition, many antigens expressed by the parasite are highly polymorphic within the same host, adding, if possible, more complexity to the already difficult process of antigen identification [8]. Another major challenge to vaccine development is financial costs. In 2020, funding for malaria-related research and development surpassed US$ 619 million, with an average annual R&D investment of US$ 851 million still needed in the period of 2021-2023 [53]. In recent years, Gavi, the Vaccine Alliance has donated US$155 million to support the introduction, procurement, and delivery of the malaria vaccine for Gavi-eligible countries in sub- Saharan Africa [53,54]. However, this effort needs to be extended to other countries, especially in sub-Saharan Africa and Asia where malaria afflicts a significant portion of the population.
The development of a successful malaria vaccine has suffered setbacks over the years due to several factors which are not limited to the intricate biology of the Plasmodium species and the way it interacts with the vector, mosquito and the definitive host, man. Several attempts have led to the discovery of the RTS, S vaccine which has so far shown greater promise than any other malaria vaccine candidate. While we await the outcome of the pilot implementation of the RTS, S, it is imperative to reflect on the efficacy of the vaccine from its previous clinical trials which evidently leaves room for improvement if we must attain total elimination of malaria in the nearest future.
We declare that no economic interest or any conflict of interest exists in the publication of this manuscript.


