Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Njila HL1*, Naanmiap D1 and Ombugadu A2
Received: June 28, 2022; Published: July 07, 2022
*Corresponding author: jila HL, Department of Science Laboratory Technology, University of Jos, P.M.B. 2084 Jos, Plateau State, Nigeria. E-mail: njilahl@gmail.com; Mobile No.: +2348163365257
DOI: 10.26717/BJSTR.2022.45.007139
Choice for suitable places by female mosquitoes to lay eggs is a key-factor for survival of immature stages. Thus a study on the assessment of water preference by gravid female mosquitoes in the selection of oviposition sites was conducted at University of Jos, Bauchi road campus, with the aim to assess water preference by gravid female mosquito species in the selection of oviposition sites. Ten 200CL green and transparent plastic buckets with a diameter of 20cm were used as oviposition containers which were placed in pairs with five different water sources namely: pond, rain, package, polluted, and tap water. They were left open in the field for a period of one month. Larvae were collected daily using pipette and rear in the insectary to adults for proper identification. Physicochemical parameters of water were monitor during the study period. A total of 1,212 mosquitoes larvae were collected from the five different water samples. There was significant difference (P<0.05) between water sources preferred for oviposition and breeding by mosquito species. The Tap water was the most preferred water source for oviposition and breeding where 435 (35.89 %) mosquito larvae were collected. However, the five water sources were favorable for oviposition and breeding by Aedes species and only three water sources (tap, rain and package water) were favorable for oviposition and breeding by Culex species. Toxorhynchites utilized only one water source (rainwater). Culex species were the highest mosquito species collected during the study. Pearson correlation showed that Aedes is positively correlated to alkalinity and negatively correlated with temperature, pH and dissolved oxygen. Culex show positive association with temperature and pH and negatively correlated with dissolved oxygen and alkalinity. Toxorhynchites is negatively correlated with temperature and alkalinity and positively correlated with pH and dissolved oxygen. Therefore, water quality is an important factor which determines whether a female mosquito will select water for oviposition and breeding and whether the resulting immature stages will successfully complete their development to adult stage.
Keywords: Water; Mosquitoes; Oviposition; Breeding; Sites
Selection of suitable sites is critical step in the life history of mosquitoes. This is a process whereby individuals select and occupy a non-random set of aquatic habitats. Habitat selection is of major importance for the interpretation of spatial and temporal distributions of populations, and for understanding intra and interspecific relations that influence the abundance of individuals [1]. Choice for suitable places by female mosquitoes to lay eggs is a key-factor for survival of immature stages [2]. Characteristics of desired breeding sites vary with mosquito species. Anopheles mosquitoes which transfer malaria typically breed outdoors in a natural occurring freshwater habitat such as pond, pool and marshes, Aedes aegypti, the primary vector of dengue is adapted to an urban environment. Thus, they primarily breed in rain-filled, man-made habitats such as household containers and discarded tyres, and there may be several breeding site per household [3]. Water conditions, location, presence of larval food, location, and shades are the determining factors of breeding sites of dengue vectors [4]. Gravid female mosquitoes may utilize the habitat with close proximity to the homestead for oviposition as an evolutionary strategy for energy conservation [5]. The attraction of oviposition site for malaria vector mosquitoes is dependent upon a number of physical and chemical factors. Many aspects of mosquito behaviour, including host location and oviposition are mediated by volatile semiochemicals.
So far, volatile compounds identified as oviposition attractants for mosquitoes include phenol, 4-methyl phenol, 4-ethyl phenol, indole, skatole, and P-cresol from hay infusion; 3-carene, α-terpinene, α-copaene, α-cednene and d-cardine released by copepods; Alcohol and terpenoids including p-cresol from plants; ethyl acetate and hydrocarbon substance probably released by filamentous algae; 3-methyl-1-butanol identified from bacteria [6]. Likewise, extracts from certain aquatic plant species exhibit oviposition repellent and deterrent properties. This may explain the absence of mosquito larvae in habitats where these plants are abundant. For example, 1, 8-cineole is a monoterpenoid found in oil extracts from Hemizonia fitchii. The 1, 8-cineole is not larvicidal, but is a highly effective oviposition repellent [7]. Biotic (flora and fauna) and abiotic (chemical and physical) factors play a significant role in larval habitat preference by both Culex species and Anopheles species mosquitoes. Thus, such factor should be taken into consideration when designing integrated vector control programme [8]. Ecology and distribution of various mosquito species is important in the determination of mosquito vector abundance and associated diseases prevalence [9,10]. Despite several research works on mosquito worldwide; more information is needed on the water preference for oviposition by the gravid female mosquito. Hence this study will provide relevant information and understanding of the most preferred water source for breeding by gravid female mosquito species, and the physicochemical factors that influence oviposition by female mosquito species.
The study was conducted in the ecological station, University of Jos, Bauchi Road campus in the rainy season of September-October 2019.
Ten 200CL green and transparent plastic buckets with a diameter of 20cm were used as containers for the study. Green buckets are chosen because mosquitoes have been reported to have colour cues for oviposition such as green colour [11,12]. The transparent buckets were selected as a contrast to the green buckets and for more visibility in observing the developmental stages of mosquitoes for identification. The containers were kept in a cool shaded area in the Garden [13].
Five Discovery Sachet water (200CL) was obtained within the campus, Pond water was collected from Department of zoology Fisheries fishpond, tap water was collected within the University, Polluted water from a gutter at Dogon Dutse along Bauchi Road, and Rainwater was collected during rainfall. Using a measuring cylinder, 200 CL of each water sample was transfer in the five green containers and five transparent containers respectively. The containers were kept in pairs at the study site at a distance of 2m to each other.
Containers were examined daily for mosquito larvae. Daily oviposition and larva in each container were collected daily and recorded. The larvae were taken to the insectary for rearing to adult stages. Adult were identified to the type of mosquito genera according to keys provided by Gillies and de Meillon [14].
Water temperature (℃) in each container was monitored daily with mercury in glass thermometer and the Hydrogen ion concentration (pH) was monitored with a Hanna scientific pH meter. Dissolve oxygen, free carbon and alkalinity (mg/L) of the water samples were determine using direct titration techniques.
Daily oviposition counts were calculated as the percentage of oviposition for each container. Average temperature, pH, Dissolved oxygen, Alkalinity and free carbon ranges were calculated. T-test, ANOVA and Pearson’s correlation were used to determine water preference, species abundance and physicochemical parameters influencing oviposition. P<0.05 was regarded as an acceptable level of significance while P> 0.05 was regarded as not significant at 5%.
A total of 1,212 mosquito larvae were collected from the five different water samples. There was significant difference (One sample Test: t = 3.769, df = 4, P = 0.020) between water sources preferred for oviposition by mosquito species (Figure 1). The breakdown of the results revealed that 435 mosquito larvae representing 35.89 % were collected from tap water, 332 (29.39 %) from rainwater, 232 (19.14 %) from package water, 123 (10.15 %) from polluted water and 90 (7.14 %) from pond water (Figure 1). The five water sources were favourable for ovipostion and breeding by Aedes species. Aedes utilized all the five water sources (Rain, pond, tap, polluted and package water) for breeding. The proportion showed that tap water had 182 (32.62%), polluted 123 (22.04 %), package water 79 (17.38%), pond 90 (16.13%) and rain 66 (11.83%) Aedes species. However, only three of the water sources (tap, rain and package water) were favourable for oviposition and breeding by Culex species. The proportion revealed that rainwater had 260 (40.12%), tap 253 (39.04 %) and package water 135 (20.83 %) of Culex species. Toxorhynchites utilized only one water source (rainwater) with 6 species. ANOVA test revealed a slightly statistically significant difference between three genera utilizing the five water sources (P= 0.046, Figure 2).
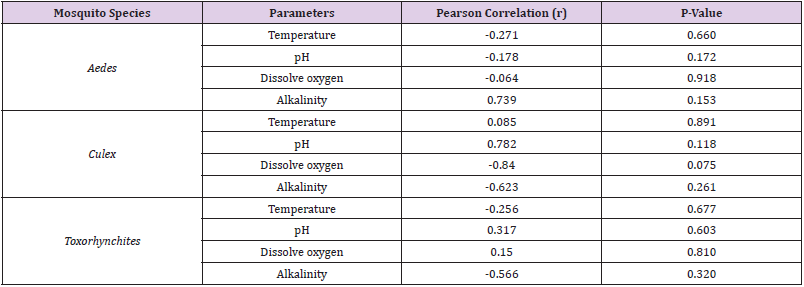
There was a significant difference (t = 102.764, df = 4, P = 0.000) in mean temperature of the water sources (Table 1). The highest mean temperature was recorded in package water (25.25±0.354) and the lowest in tap water (23.90±0.000) as shown in Table 1. There was also a significant difference (T-test: t = 67.625, df = 4, P = 0.000) in water pH across the five water sources (Table 1). The water pH recorded was slightly alkaline, the highest pH mean was recorded in tap water (7.84±0.064) and the lowest in pond water (7.23±0.205) as shown in (Table 1). There was significant difference (T- test: t = 39.060, df = 5, P = 0.000) in mean dissolved oxygen value in the five water sources Table 1. The highest dissolved oxygen was recorded in polluted water (9.50±2.404), and the lowest was recorded in Tap water (7.85±1.344) as shown in Table 1. There was significant difference (T- test: t = 3.605, df = 5, P =0.015) in the mean Alkalinity value across the five water sources (Table 1). The highest alkalinity mean values value was recorded in pond water (77.50±9.192), and the lowest was recorded in Rainwater (22.00±8.485) as shown in Table 1. There was no significant difference (T- test: t = 1.518, df = 5, P = 0.190) in the mean value of free carbon in the five water sources (Table 1). Free carbon in the five-water sample has no difference in the mean value across the five water sources (Table 1). Be that as it may, Aedes species showed positive correlation with alkalinity (r = 0.739; P = 0.153), but negatively correlated with Temperature (r = -0.271; P = 0.660), pH (r = -0.718; P = 0.172) and DO (r = -0.064; P =0.918) with no significant difference, as shown in Table 2. Culex species showed positive correlation with Temperature (r = 0.085; P = 0.891), pH (r = 0.782; P = 0.118) and not statistically significant. While negatively correlated with DO (r = -0.840; P = 0.075), Alkalinity (r= -0.623; P = 0.261) and not statistically significant as shown in Table 2. Toxorhynchites species showed positive correlation with pH (r = 0.317; P = 0.603) and DO (r = 0.150; P = 0.810), while negatively correlated with temperature (r = -0.256; P = 0.677) and Alkalinity (r = -0.566; P = 0.320) and all are not statistically significant as shown in Table 2.
Table 2: Pearson’s correlation (r) coefficient of Physico-chemical properties of breeding water and larva density of Aedes, Culex and Toxorhynchites s in the five water sources.
Mosquitoes breed in wide range of aquatic habitats with different types of water that are known to be specific for certain species [15]. Water quality is an important factor which determines whether a female mosquito will select water for breeding and whether the resulting immature stages will successfully complete their development to adult stage [16]. In this study tap water was identified as the most preferred water for oviposition by gravid female mosquito. This is an indication that it contains some substances which served as attractant for oviposition by gravid female mosquitoes and this is of concern to the public drinking water. It is also an indication of poor level of water treatment before the water is supplied to the public [17]. This study also revealed that Aedes species utilized all the five water sources for breeding, probably because they preferred to breed in man-made or domestic containers. This is in agreement with the study conducted by Jacklyn et al. (2011) [18] and Chareonviriyaphap et al. [19] where they observed that Aedes aegypti preferred to breed primarily in water storage containers, especially domestic containers. However, Culex species utilized three water sources namely: Rain, Tap and Package water in this study. This is probably because of their preference for polluted water which favors their feeding requirements. This differed from study conducted by Okwa and Savage [20] where Culex utilized all the water sources for its oviposition. Toxorhynchites species, the larviparous mosquito were also identified in the course of the study and their predatory larvae were observed to feed upon the larvae of Aedes and Culex species. This is consistent with the findings of Larissa and Alison [21] working on the Biology of Toxorhynchites mosquito as potential bio-control agent, suggested the use of Toxorhynchites as an alternative biological agent for mosquito vectors, since their female always seek the same aquatic habitat to lay their eggs with other mosquito vector species without being manually introduce like other biological agents.
Prevailing physicochemical parameters in habitat are important factors for survival and development of mosquitoes [20]. The temperature recorded in this study was in the range of 23.90℃ to 25.25℃, this result fall within the temperature range recorded by Amerasinghe et al. [22] who reported a temperature of 28℃ as ideal for mosquito oviposition. While Daniel et al. [23] reported a temperature of 24-30℃ as suitable for incubation of Anopheles gambiae. A pH range of 7.23 - 7.84 was recorded in this study, which fall within the pH range of 6.8 – 8.5 which was reported by Osta et al. [24] as the ideal pH range for mosquito breeding on the water surface. While Gopalakrishnan et al. [25], reported a pH range of 7.5 to 8.02 where Aedes aegypti larvae are found to survive at maximum. The result obtained for dissolved oxygen in this study, slightly differed from what was obtained by Nazri et al. [26] who work on water quality characteristics of dengue vectors in thirteen different breeding containers in Subang Jaya, Malaysia to know the specific water quality that will support the breeding of dengue virus, with a dissolved oxygen value in the range of 5.72 to 7.92 mg/L. while this study recorded a dissolved oxygen value of a range 7.85 to 9.50mg/L. Alkalinity obtained from this study was in the range of 22 to 77 mg/L, this result is not in agreement with was obtained by Nikookar et al. [27], where the Alkalinity result obtained in the different mosquito larval habitat was in the range of 168 to 500 mg/L. Pearson’s correlation result obtained from this study show that Aedes is positively correlated in relation to Alkalinity while negatively correlated with Temperature, pH and Dissolve oxygen.
The result of correlation between Aedes with pH and dissolve oxygen vary from the result obtained by Afroza et al. [28], where pH and dissolved oxygen were positively correlated with Aedes larva density. But the negative correlation of Aedes with temperature agrees with their result, where Aedes was negatively correlated with temperature in all the breeding sites in their study. Culex show positive association with temperature and pH and negatively correlated with dissolved oxygen and alkalinity. Correlation of Culex with pH agreed with the previous study conducted by Tadesse et al. [29]. Toxorhynchites is negatively correlated with temperature and alkalinity and positively correlated with pH and dissolved oxygen.
The result obtained from this study clearly shows that tap water is the most preferred water for oviposition by gravid female mosquito. The number of mosquito larvae collected varied significantly across the five water sources and Culex species was the most abundant species collected. While Aedes species breed in all the water sources and were less in abundance compared to Culex species. The least species collected was Toxorhynchites and breed in only one water source. Data obtained from physicochemical parameter, (Temperature, pH, dissolved oxygen, Alkalinity and free carbon), showed either positive or negative correlation with Culex, Aedes and Toxorhynchites. This study postulated that physicochemical parameters exert a significant influence on larval occurrence and thus provide important information that can be of use in the development of an effective vector control strategies to reduce potential breeding habitats and occurrence of mosquitoes.
All authors contributed to the design of the study. NHL suggested the research topic. NHL and ND wrote the first draft of the manuscript and OA reviewed the final draft and performed the analysis. All authors read and approved the article for publication.
NHL would like to thank Dr. Mafuyai Godwin of the Department of Chemistry, University of Jos, Nigeria for making available chemical reagents used for chemical analyses of water samples.
The authors have no funding or financial relationships.
The authors declare that there is no conflict of interest.


