Abstract
A simple method for measurement of serum antibodies against Helicobacter pylori (H. pylori) by indirect fluorescent antibody (IFA) technique using latex particles was developed. Sera were collected from Mongolian gerbils inoculated with H. pylori. Serum titers were measured using the IFA technique coupled with solubilized H. pylori. Fluorescence findings for latex particle-coupled soluble H. pylori were only confirmed in samples containing antibodies against H. pylori. Serum IgM and IgG titers increased after inoculation with H. pylori, and serum IgM titers increased before IgG titers. It is therefore possible to confirm infection at early stages using anti-IgM antibody as a secondary antibody. Moreover, the IFA technique needed only 10 μl of serum from Mongolian gerbils. This method will be useful for confirming H. pylori infection in individual animals.
Keywords: IFA; H. pylori; Serum Antibody Titer; Mongolian Gerbils
Abbreviations: IFA: Indirect Fluorescent Antibody; FITC: Fluorescein Isothyocyanate; PBS: Phosphate-buffered Saline; VT: Verotoxin
Introduction
Helicobacter pylori (H. pylori), first identified by Marshall and Warren, is a gram-negative bacterium found on the luminal surface of the gastric epithelium [1]. Mongolian gerbils infected with H. pylori reflect human gastric disease caused by H. pylori [2- 4]. Mongolian gerbils are considered to be a suitable experimental model for H. pylori infection [3,5,6]. Establishment of infection is confirmed by culture using the removed stomach from Mongolian gerbils in a satellite infection group before infection experiments. However, it is impossible to ensure that all Mongolian gerbils used in the study are infected, even if all Mongolian gerbils in the satellite group are confirmed to be infected with H. pylori. Measurement by indirect immunofluorescence assay (IFA) has high specificity and is a simple method for obtaining antibody titers. We have already reported that the antibody titer of bovine immune colostral antibody against verotoxin (VT) 2 can be measured using latex particles coupled with VT2 [7]. The aim of this study was to evaluate whether infection with H. pylori can be confirmed in individual Mongolian gerbils by IFA.
Materials and Methods
Animal Experiment
Five Mongolian gerbils were purchased from Japan SLC, Inc. (Hamamatsu, Shizuoka, Japan). Mongolian gerbils under fasting for 18 hours were orally inoculated with H. pylori adjusted to 5 × 107 CFU/ml. Blood was collected from the jugular vein under anesthesia before inoculation and at 1-week intervals until 8 weeks after inoculation. Sera were obtained by centrifugation (9,200 × g, 15 minutes). The present animal experiments were approved by the Institutional Animal Care and Use Committee of Azabu University.
IFA Technique
Latex particles of 6.0 μm in diameter (Polyscience Inc., Warrington, PA) were used. Soluble antigens of H. pylori were bonded with the latex particles according to the method of Kuribayashi, et al. [8]. Latex particles sensitized with H. pylori soluble antigen were diluted fifty times. Diluted latex particles (10μl) were smeared on one well of the slide glass (Matsunami Glass Ind., Ltd., Osaka, Japan), followed by drying at room temperature. Analyte sera were diluted between 5 and 80 times with phosphate buffered saline. Analyte sera (10 μl) was smeared on a slide glass (Matsunami Glass Ind., Ltd.) and reacted under a moist environment for 1 hour. Slide glass was washed with phosphatebuffered saline (PBS, pH7.2) for 5 minutes, and was then washed with PBS containing 5% glycerin. Finally, the slide glass was dried at room temperature after rinsing with distilled water for 3 minutes. Fluorescein isothyocyanate (FITC)-labeled goat anti-mouse IgG antibody (American Qualex International Inc., San Clemente, CA) or FITC-labeled goat anti-mouse IgM antibody (American Qualex International Inc.) was smeared onto a slide glass and reacted for 1 hour at room temperature. The slide glass was washed using the same method mentioned above and dried at room temperature. Antibody titers were determined at the maximum dilution before fluorescence was no longer observed.
Results
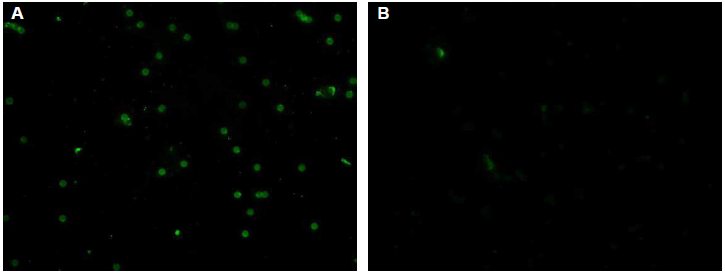
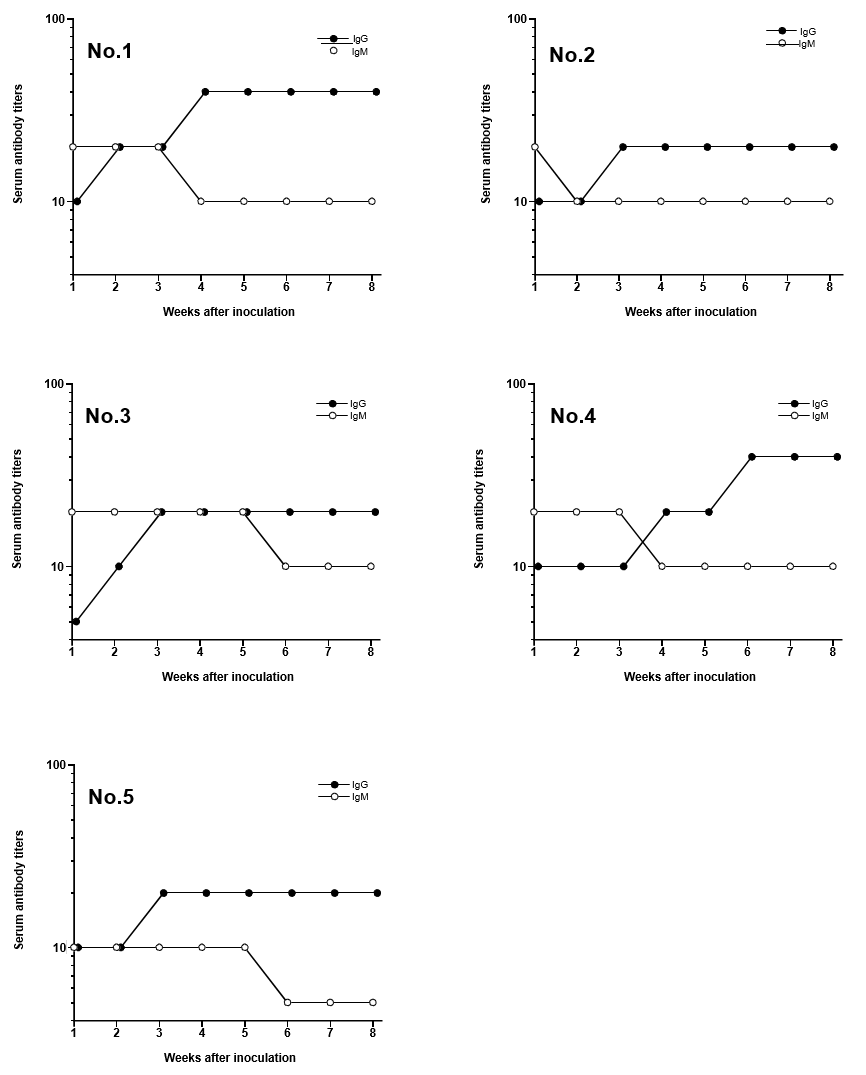
Typical negative and positive fluorescent observations of latex particles with adsorbed H. pylori soluble antigen after reaction with serum with or without antibodies against H. pylori are shown in (Figure 1). No fluorescence was observed with serum lacking antibodies against H. pylori. On the other hand, particles coupled with H. pylori soluble antigen reacted with serum antibodies against H. pylori, showing fluorescence. Serum IgG and IgM titers increased after inoculation with H. pylori (Figure 2).
Figure 2: Changes of serum IgM and IgG titers in Mongolian gerbils after inoculation of Helicobacter pylori Each number was represented the animal number.
Discussion
Mongolian gerbils are essential experimental animals for evaluating H. pylori infection [2,4]. It is therefore important to confirm the establishment of infection in individual animals to ensure the accuracy of experimental infection studies. The IFA technique using latex particles with soluble antigens from H. pylori was therefore used to evaluate serum titers in Mongolian gerbils after inoculation. Elevation of serum antibodies was found to be correlated with infection by H. pylori in Mongolian gerbils [9]. Serum IgM titers increased before IgG titers at 1 week after inoculation with H. pylori. It is therefore possible to confirm infection at early stages using anti-IgM antibody as a secondary antibody. Only 10 μl of serum was needed to measure serum titers using the IFA technique. Therefore, all Mongolian gerbils were confirmed to be infected with H. pylori using the IFA technique in an H. pylori infection experiment, and only required the collection of a very small quantity of blood from each animal. Thus, only Mongolian gerbils confirmed to be infected with H. pylori would be used in the infection study, thus improving experimental precision. Moreover, it would be unnecessary to confirm H. pylori infection in a satellite group, which would reduce the number of experimental animals.
Conclusion
In summary, serum titers in individual Mongolian gerbils could be measured using the IFA technique. Establishment of infection with H. pylori in each Mongolian gerbil could therefore be confirmed, and this approach will improve the accuracy of experimental H. pylori infection studies.
Acknowledgment
None.
References
- Warren JR, Marshall BJ (1983) Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1(8390): 1311-1314.
- Hirayama F, Takagi S, Yokoyama Y, Iwao E, Ikeda Y (1996) Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol 31: 450-454.
- Romero Gallo J, Harris EJ, Krishna U, Washington MK, Perez Perez GI, et al. (2008) Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest 88(3): 328-336.
- Tsukamoto T, Toyoda T, Mizoshita T, Tatematsu M (2013) Helicobacter pylori infection and gastric carcinogenesis in rodent model. Semin Immunopathol 35: 177-190.
- Konturek PC, Konturek PC, Brzozowski TG (2006) Gastric cancer and Helicobacter pylori J Physiol Pharmacol 57: 51-65.
- Zhang S, Moss SF (2012) Rodent models of Helicobacter inflammation, and disease. Methods Mol Biol 921: 89-98.
- Seita T, Kuribayashi T, Yamaguchi S, Furuhata K, Yamamoto S (2014) New application of indirect fluorescent antibody (IFA) technique using latex particles coupled with verotoxin 2 from Escherichia coli O157:H7 in order to determine colostral antibody titers in immunized dairy cows. J Immunoassay Immunochem 35(3): 314-321.
- Kuribayashi T, Seita T, Honjyo T, Kawato K, Takada K, Yamamoto S (2013) Determination of antibody titers against soluble antigen by indirect fluorescent antibody (IFA) assay using latex particles coupled with soluble antigen: A preliminary study. J Immunoassay Immunochem 34(1): 39-48.
- Kawato K, Seita T, Kuribayashi T, Takagi Y, Matsuda M, et al. (2014) Optimization of Infectious Conditions with Helicobacter Pylori in the Infection-highly Resistant Mongolian Gerbils Supplied in Japan. Intern J Appl Res Vet Med 12(3): 248-255.

 Short Communication
Short Communication