Abstract
Cells need to respond and adapt to the changes of their surrounding physical environment for survival. On the other hand, autophagy is a catabolic mechanism for cells to cope with stress. But the relationship between cell autophagy and surrounding physical environment is poorly understood. In the past decade, accumulating number of literatures started to focus on the role of autophagy in cell mechanobiology, especially in myocardial cells, chondrocytes and endothelial cells. The results demonstrated that mechanical factors may lead to autophagy through PI3K-AKT-mTOR, oxygen free radical, AKT-FoxO and other pathways. The autophagic response of cells is a protective mechanism for cells to cope with their surrounding physical environment
Keywords: Stress; Pathogen; Autophagy; Lysosome; Blood Flow; Eukaryotic Cells; Mechanical Stimuli; Morphology
Introduction
Autophagy means self-eating, and it is a natural and regulated
mechanism of cells that remove unwanted or dysfunctional
components, especially under external stimuli, such as pathogen
invasion, starvation and hypoxia [1,2]. It allows orderly degradation
and recycling of cellular components and exists in all eukaryotic
cells. Autophagy is a highly conserved process that catabolizes
intracellular components to maintain energy homeostasis and to
protect cells against stress [3,4]. When autophagy occurs, the cell will
first produce an isolation membrane structure called phagophore,
which has a double-layer membrane structure. The phagophore
will wrap the pathogens and damaged organelles in the cell through
extension. After that, the autophagosome double membrane
structure is formed to close the vesicle, and then the lysosome and
the autophagosome are fused together to form the autolysosome.
Finally, the fusion process is completed, and a monolayer structure
is formed to degrade unnecessary cellular components [5,6].
After being degraded, the final autophagy lysosome structure will
transport the degraded product out of autolysosome through the
transfer protein on the membrane surface and supply it to the cell
for energy or material recovery and reuse.
Through this process, the cells can respond to the lack of
nutrients and energy under starvation conditions, and to achieve
the self-supply of substances and energy. At the same time, it also
can degrade some toxic substances to prevent their damaging effects
to normal cells [7,8]. The process of autophagy is complicated, and
there are still many details that we have not grasped. Other the
other hand, cells and tissues within the human body are always in
a certain physical environment [9]. For example, skeletal muscle is
subjected to stretch; joint cartilage is under pressure; blood vessel
endothelium cells are facing blood flow shear stress. Cells are able
to respond to their surrounding mechanical environments, in which
mechanical stimuli modulate cell proliferation, differentiation,
morphology, migration and extracellular matrix production, as
well as other physiological functions [9,10]. Cells need to adapt to
their surrounding environments. Poor adaptation usually leads to
pathological phenomena and occurrence of diseases. For example,
myocardial hypertrophy is due to the heart muscle cells coping with
long-term blood flow pressure or overloaded capacity. In serious
cases, it may develop into heart failure [11].
Autophagy is an important mechanism for cells to cope with mechanical stimuli. Flow shear stress can promote endothelium cell autophagy activity and maintain its normal physiological activities. Abnormal stress can lead to poorly adapted pathological phenomena in tissues and cells. Autophagy is also closely related to fat, failed heart, degenerative lesions of joint cartilage and intervertebral discs [12,13]. Thus, in recent years, the influence of mechanical factors on cell autophagy has aroused great interests in researchers. This paper summarizes the effects of mechanical stimuli on the autophagy activity of different cells and tissues as well as relevant molecular mechanisms, and subsequently may provide theoretical basis for autophagy-related diseases (Figure 1).
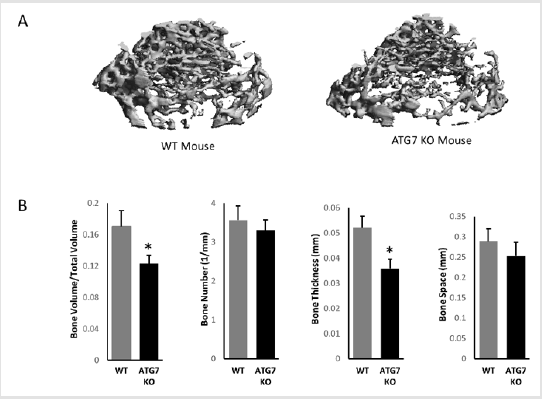
Figure 1: Bone structures of wild type and autophagy deficient mice (8 weeks of age). (A) micro-CT images; (B) Quantified parameters of trabecular bone of wild type and autophagy deficient mice. (n = 4, *p,0.05). Error bars represent SEM.
Introduction
Effects of Mechanical Stimuli on Myocardial Cell Autophagy
The heart is responsible for blood supply and it is always under
strong blood flow pressures. If the myocardium is overloaded for
a long time, left ventricular hypertrophy will occur. The symptoms
include abnormal contraction, arrhythmia, abnormal energy
metabolism and heart failure [14]. In this situation, the expression
and activity of proteasome in heart muscles are significantly
enhanced due to autophagy in ventricular hypertrophy [15]. Zhu,
et al. [12] discovered for the first time the role of autophagy in
pressure-overload-induced cardiac hypertrophy [12]. They used
autophagy-reporter mice to confirm that pressure overload would
cause increased autophagy activity continuously in cardiomyocytes.
The increase in autophagy can be maintained for at least 3 weeks.
Furthermore, other researchers have found that the autophagy
activity of cardiomyocytes is resulted from stress due to blood
pressure [16-18]. They also found that knocking out beclin1 can
decrease the autophagy activity induced by blood pressure. On the
other hand, overexpression of beclin1 in mice leads to the opposite
effects. Another study using atg5 knockout mice study confirmed
the previous results [19]. Hariharan et al. [20] loaded primary rat
cardiomyocytes cultured in vitro with a strain of 20% strain for
36 h, and found that p62 degradation was significantly reduced,
indicating an increase in autophagy activity [20]. Lin et al. [17]
applied a strain of 20% to primary rat cardiomyocytes and COS7
cells in vitro for 48 hours. Subsequently, expression of LC3-II in
cells increased, and the number of autophagosomes also increased
significantly [17].
The effects of mechanical stimuli on cardiomyocyte autophagy
are closely associated to proteasome activities. Pressure overload
leads to an increase in the amount of misfolded protein in
cardiomyocytes, and these proteins gradually gather around the
nucleus to form aggregates, which are recognized and degraded
by the autophagy system [16]. Pressure may cause accumulation
of ubiquitinated proteins, aggregate formation and increased
autophagy activity in left ventricular myocardial cells. Inhibiting
cardiomyocyte proteasome activity in vitro makes ubiquitinated
protein accumulate, and subsequently triggers autophagy. On the
other hand, inhibiting autophagy activity of cardiomyocytes can
effectively enhance the size and number of protein aggregates.
PI3K-AKT-mTOR pathway plays important role in regulating cell
autophagy [21]. AKT regulates cardiomyocyte autophagy through
its downstream transcription factor FoxO (forkhead box, class O)
protein family, which can promote autophagy activity [22]. When
cardiomyocytes are under mechanical stimulation, the effects of
on FoxOs are inhibited. Subsequently, activated FoxO1 leads to
cardiomyocyte ubiquitination and lysosome activities, and further enhances autophagy and protein degradation [23]. Additionally,
FoxO3 can activate downstream Bcl2 family member Bnip3, which
inhibits the binding of Bcl2 and Beclin1 and promotes autophagy.
While pressure overload causes increased autophagy and
hypertrophy of cardiomyocytes, there was also an increase
of angiotensin II level in plasma and myocardium, and blood
angiotensin II can promote autophagy activity in cells [24]. During
pressure overload, expression of angiotensin II, phosphorylated
PKCb1 and ERK1/2 are all increase significantly in cardiomyocytes
[25]. Inhibiting anyone of them leads to inhibition of cardiomyocyte
autophagy, suggesting the involvement of the AngII-PKCb1-
ERK1/2 pathway during this process. Angiotensin II can inhibit
the expression of miR-34a in cardiomyocytes, which binds to
Atg9A to inhibit autophagy activity. Angiotensin II type I receptor
(AT1) mediates a variety of regulation of angiotensin II and
plays an important role in myocardial hypertrophy induced by
mechanical stress [26]. AT1 can mediate stress-induced autophagy
independently of angiotensin II and p38 MAPK is involved in
the process. However, ERK1/2 and JNK are not involved in the
regulation of autophagy, in which AT1 plays an important role
through PI3K-AKT signal pathway [17].
Cardiomyocyte autophagy is affected by mechanical stimuli and
serves as a protective mechanism for cells to adapt their physical
environments, subsequently to maintain their normal functions.
The molecular mechanism of how mechanical stimuli induce
cardiomyocyte autophagy is still unclear and needs further studies.
Effects of Mechanical Stimuli on Chondrocyte Autophagy
Articular cartilage is a soft connective tissue that covers the subchondral bone in a diathrodial joint. It is porous and filled with synovial fluid, which can flow in and out during motion and serves as a lubricating material to the joint. Mechanical stimuli have profound effects on the function of articular cartilage. Physiological level mechanical stress is beneficial for cartilage to maintain normal functions, while abnormal mechanical stress may result in chondrocyte death, extracellular matrix degradation and mineralization, osteoarthritis and other cartilage degenerative diseases [27]. During abnormal conditions, autophagy plays an important role in cartilage responses. Carames et al. [28] first studied the effect of mechanical shock on chondrocyte autophagy. They applied 40% strain mechanical shock to cartilage tissues of cattle and human and found that the survival rate of cells was significantly reduced. They also found that sulfated glycosaminoglycans in extracellular matrix were gradually lost, and the expression of autophagy marker LC3-II in chondrocytes was increased in 24 hours. But the expression levels of ULK1, beclin1 and LC3-II were significantly reduced at 48h and 96h. Pretreatment with autophagy inducer rapamycin can enhance autophagy activity, reduce cell death and the loss of sulfated glycosaminoglycans, indicating that autophagy may play a protective role in the early stage of chondrocytes subjected to mechanical shock [28,29].
Ma et al. studied the effect of excessive mechanical pressure
(1 MPa) on the autophagy of the colloidal nucleus pulposus cells
(NP cells) in the center of the intervertebral disc and found that
compared with the control group, the cell survival rate of the
pressurized group was significantly reduced, and the ratio of cells
that underwent autophagy increased significantly, and autophagy
activity in cells also increased [13]. At the same time, oxygen free
radicals accumulated in the cells during this process, suggesting
that NP cells may activate autophagy through the oxygen free
radical signaling pathway to cope with excessive mechanical stress.
They also found that treating cells with autophagy inhibitor 3MA
can significantly reduce the autophagy of NP cells. But at the same
time, the occurrence rate of apoptosis increased significantly.
In another study, researchers investigated the effects of
intermittent cyclic mechanical stretch on the calcification and
autophagy of endplate chondrocytes. They found that chondrocyte
autophagy activity was significantly increased on the 5th day,
but significantly decreased on the 10th and 20th days [30].
Thus, short-term intermittent cyclic mechanical tension can
promote chondrocyte autophagy, while long-term intermittent
cyclic mechanical tension can inhibit autophagy. They also found
that chondrocyte autophagy can protect cell from calcification.
In chondrocytes, autophagy is a self-protection mechanism to
respond to mechanical stimuli. When cartilage is under pressure,
chondrocyte autophagy will increase in a short time. But if the
stimulation lasts too long, the level of autophagy begins to decrease.
Insufficient protection is gradually replaced by other mechanisms,
such as apoptosis. Additionally, excessive mechanical stimulation
may cause unregulated cell death.
Effects of Mechanical Stimuli on Endothelial Cell Autophagy
Endothelial cells are an important part of blood vessels, and they
play an important role in maintaining the stability of blood vessel
structure and functions. Under normal physiological conditions,
endothelial cells are mainly affected by three mechanical factors:
pressure, circumferential stretch and flow shear stress. Among
them, flow shear stress affects gene expression, proliferation,
migration, morphogenesis and adhesion of endothelial cells as
well as their permeability and inflammation [31]. Autophagy
is also affected by flow shear stress in order to maintain normal
homeostasis in endothelial cells. It regulates vascular endothelial
cell eNOS and ET-1 expression induced by laminar shear stress
[32]. Additionally, different types and sizes of flow shear stress
have different effects on endothelial cell autophagy. Laminar flow
shear stress of 1.2Pa or 2Pa promoted endothelial cell autophagy
activity, while the pathological type of oscillating flow does not
promote autophagy. The magnitude of laminar shear stress also
affects endothelial cell autophagy. Laminar shear stress of 0.4Pa
didn’t promote endothelial cell autophagy [33]. Their results are
consistent with previous study that the laminar shear stress of 0.5- 1.5Pa promoted endothelial cell autophagy [32]. However, Ding et
al. [34] showed that shear stress of 0.3Pa can activate endothelial
cell autophagy. When the magnitude of the flow shear stress
increased to 3Pa, the effects on endothelial cell autophagy gradually
disappeared [34].
Besides inducing endothelial cell autophagy, flow shear stress
also enhanced expression of nitric oxide synthase and inhibited
the expression of endothelin1 which play important roles in
maintaining endothelial cell functions and vasoconstriction [35].
The use of autophagy inhibitors and inducers confirmed that
autophagy modulates expression of nitric oxide synthase and
endothelin1. In inflammatory states, the effects of flow shear
stress on endothelial cell autophagy are enhanced [34]. When
the autophagy pathway is blocked, flow shear stress can promote
cytokine MCP-1 and interleukin-8 expressions, suggesting that
endothelial cell autophagy promoted by flow shear force may
have an anti-inflammatory effect. In addition, the flow shear
stress promotes endothelial cell autophagy and is related to the
intracellular oxidation-antioxidant balance [35].
The accumulation of oxygen free radicals was detected in
endothelial cells stimulated by flow shear stress [33,35]. Sirt1,
an intracellular sensor of redox activity, is induced by flow shear
stress. It can sense the production and accumulation of oxygen free
radicals which may induce autophagy. In addition, upregulation
of Sirt1expression activates FoxO1 and FoxO3, and subsequently
induces the autophagy response. The expression of LOX-1 was also
up-regulated, and LOX-1 could also activate autophagy through
oxygen free radicals [34].
Effects of Mechanical Stimuli on Autophagy of other Cells and Tissues
Besides heart, cartilage and blood vessel, mechanical stress also can induce autophagy in skeletal muscle, and has great impact on skeletal muscle functions. Gumucio, et al. [36] found that autophagy related Vps34 and Beclin1 gene expression increased in rat skeletal muscle cells in rotator cuff tear rat model, indicating that autophagy was induced when skeletal muscle was under shear stress [36]. Ning et al. [37] obtained the similar results and they also found that proteoglycan inhibited the up-regulation of skeletal muscle autophagy [37]. In addition to macroautophagy, chaperonemediated autophagy is also involved in the influence of mechanical factors on skeletal muscle. Researchers found that after skeletal muscle cells were stimulated by stretch, chaperone-mediated autophagy was activated in order to maintain cell stability as a mechanism for transducing mechanical signals. Chaperonemediated autophagy was also responsible for activating a series of pathways and regulating skeletal muscle basic functions such as migration, adhesion, and proliferation. They also found that chaperone-mediated autophagy is an adaptive mechanism for skeletal muscle to resist acute exercise and repeated mechanical stimulation [38,39].
The autophagy of nerve cells is also affected by mechanical stress. The research in this area mainly revolves around the mechanical damage of nerve cells. When nerve cells are mechanically damaged (scratched), the level of autophagy is increased through the mTOR pathway. Additionally, compression injury can also increase the level of autophagy of nerve cells. In the early stage of cell damage, autophagy can inhibit apoptosis and protect nerve cells [40]. The mTOR pathway regulates the expression of Bax and Bcl-2 by inhibiting apoptosis. In addition to macroautophagy pathways, chaperone mediated autophagy also participates in the process of nerve cells coping with mechanical damage [41]. In addition to the aforementioned cells and tissues, there are a small number of reports on the effects of mechanical stress on autophagy of fibroblasts, podocytes and bone cells. Due to the limitation of information, we will not review these reports in this article.
Conclusion
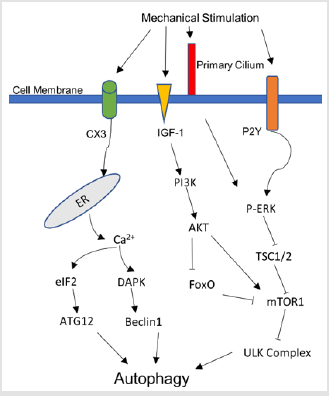
In general, autophagy is a self-protective response of cells in response to mechanical stimuli. When subjected to mechanical stress at the physiological level, cells up-regulate the level of autophagy to maintain normal cell homeostasis, function and survival. Mechanical factors may cause autophagy through PI3KAKT- mTOR, oxygen free radical, AKT-FoxO and other pathways. The influence of mechanical factors on autophagy is closely related to many diseases, such as myocardial hypertrophy, atherosclerosis, cartilage degenerative diseases, and spinal cord injury. The current research has just started, and the influence of mechanical factors on autophagy remains to be clarified, and its regulatory mechanism is unclear. With further research to elucidate of the mechanism, it will help to understand the pathogenesis of these diseases and provide better prevention and treatment methods (figure 2).
Conflicts of Interest
The authors declare that they have no conflict of interests.
Funding Statement
This work was funded by Grant #11672078 from the National Natural Science Foundation of China.
References
- Ichimiya T, Yamakawa T, Hirano T, Yokoyama Y, Hayashi Y, et al. (2020) Autophagy and Autophagy-Related Diseases: A Review. Int J Mol Sci 21(23): 8974.
- Dyshlovoy SA (2020) Blue-Print Autophagy in 2020: A Critical Review. Mar Drugs 18(9): 482.
- Klionsky DJ, SD Emr (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497): 1717-1721.
- Fang Y, J Tan, Q Zhang (2015) Signaling pathways and mechanisms of hypoxia-induced autophagy in the animal cells. Cell Biol Int 39(8): 891-898.
- Reggiori F (2006) Membrane origin for autophagy. Curr Top Dev Biol 74: 1-30.
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin J, Abeliovich H, et al. (2016) Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 12(1): 1-222.
- D'Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6): 582-592.
- Eskelinen EL (2019) Autophagy: Supporting cellular and organismal homeostasis by self-eating. Int J Biochem Cell Biol 111: 1-10.
- Romani P, Lorea Valcarcel-Jimenez, Christian Frezza, Sirio Dupont (2020) Crosstalk between mechanotransduction and metabolism. Nat Rev Mol Cell Biol 20: 22-38.
- Liu X, F Nakamura (2020) Mechanotransduction, nanotechnology, and nanomedicine. J Biomed Res p. 1-10.
- Krueger W, Bender N, Haeusler M, Henneberg M (2020) The role of mechanotransduction in heart failure pathobiology-a concise review. Heart Fail Rev.
- Zhu H, Tannous P, Janet L Johnstone, Kong Y, John M Shelton, et al. (2007) Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest 117(7): 1782-1793.
- Ma KG, ZW Shao, SH Yang, J Wang, BC Wang, et al. (2013) Autophagy is activated in compression-induced cell degeneration and is mediated by reactive oxygen species in nucleus pulposus cells exposed to compression. Osteoarthritis Cartilage 21(12): 2030-2038.
- Rothermel BA, JA Hill (2008) Autophagy in load-induced heart disease. Circ Res 103(12): 1363-1369.
- Depre C, Wang Q, Yan L, Hedhli N, Peter P, et al. (2006) Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation 114(17): 1821-1828.
- Tannous P, Zhu H, Nemchenko A, Jeff M Berry, Janet L Johnstone, et al. (2008) Intracellular protein aggregation is a proximal trigger of cardiomyocyte autophagy. Circulation 117(24): 3070-3078.
- Lin L, Liu X, Jianfeng Xu, Liqing Weng, Jun Ren, et al. (2015) High-density lipoprotein inhibits mechanical stress-induced cardiomyocyte autophagy and cardiac hypertrophy through angiotensin II type 1 receptor-mediated PI3K/Akt pathway. J Cell Mol Med 19(8): 1929-1938.
- Fu L, Wei CC, Powell PC, Bradley WE, Collawn JF, et al. (2015) Volume overload induces autophagic degradation of procollagen in cardiac fibroblasts. J Mol Cell Cardiol 89(Pt B): 241-250.
- Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, et al. (2007) The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med 13(5): 619-624.
- Hariharan N, Ikeda Y, Hong C, Alcendor R, Usui S, et al. (2013) Autophagy plays an essential role in mediating regression of hypertrophy during unloading of the heart. PLoS One 8(1): e51632.
- Hill JA (2011) Autophagy in cardiac plasticity and disease. Pediatr Cardiol 32(3): 282-289.
- Hariharan N, Maejima Y, Nakae J, Paik J, DePinho RD, et al. (2010) Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res 107(12): 1470-1482.
- Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, et al. (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117(3): 399-412.
- Porrello ER, Angelo D'Amore, Claire L Curl, Andrew M Allen, Stephen B Harrap, et al. (2009) Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension 53(6): 1032-1040.
- Weng, LQ, Zhang Wb, Ye Y, Yin Pp, Yuan J, et al. (2014) Aliskiren ameliorates pressure overload-induced heart hypertrophy and fibrosis in mice. Acta Pharmacol Sin 35(8): 1005-1014.
- Yue H, Li W, Desnoyer R, Sadashiva S Karnik (2010) Role of nuclear unphosphorylated STAT3 in angiotensin II type 1 receptor-induced cardiac hypertrophy. Cardiovasc Res 85(1): 90-99.
- Zhao Z, Li Y, Wang M, Zhao S, Zhao Z, et al. (2020) Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J Cell Mol Med 24(10): 5408-5419.
- Caramés B, Taniguchi N, Seino D, Blanco FJ, Darryl D'Lima, et al. (2012) Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum 64(4): 1182-1192.
- Nogueira-Recalde U, Lorenzo-Gomez I, Francisco J Blanco, María I Loza, Grassi D, et al. (2019) Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. E Bio Medicine 45: 588-605.
- Xu HG, Yun-fei Yu, Quan Zheng, Wei Zhang, Chuang-dong Wang, et al. (2014) Autophagy protects end plate chondrocytes from intermittent cyclic mechanical tension induced calcification. Bone 66: 232-239.
- Pan S (2009) Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal 11(7): 1669-1682.
- Guo F, Li X, Peng J, Tang Y, Yang Q, et al. (2014) Autophagy regulates vascular endothelial cell eNOS and ET-1 expression induced by laminar shear stress in an ex vivo perfused system. Ann Biomed Eng 42(9): 1978-1988.
- Liu J, Bi X, Chen T, Zhang Q, Wang SX, et al. (2015) Shear stress regulates endothelial cell autophagy via redox regulation and Sirt1 expression. Cell Death Dis 6(7): e1827.
- Ding Z, Liu S, Deng X, Fan Y, Wang X, et al. (2015) Hemodynamic shear stress modulates endothelial cell autophagy: Role of LOX-1. Int J Cardiol 184: 86-95.
- Bharath LP, Mueller R, Li Y, Ruan T, Kunz D, et al. (2014) Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can J Physiol Pharmacol 92(7): 605-612.
- Gumucio JP, Davis MX, Bradley JR, Stafford PL, Schiffman CJ, et al. (2012) Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res 30(12): 1963-1970.
- Ning L, Xu Z, Furuya N, Nonaka R, Yamada Y (2015) Perlecan inhibits autophagy to maintain muscle homeostasis in mouse soleus muscle. Matrix Biol 48: 26-35.
- Ulbricht A, Felix J Eppler, Victor E Tapia, Peter F M van der Ven, Nico Hampe, et al. (2013) Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr Biol 23(5): 430-435.
- Ulbricht A, Gehlert S, Leciejewski B, Schiffer T, Bloch W, et al. (2015) Induction and adaptation of chaperone-assisted selective autophagy CASA in response to resistance exercise in human skeletal muscle. Autophagy 11(3): 538-546.
- Chen Z, Fu Q, Shen B, Huang X, Wang K, et al. (2014) Enhanced p62 expression triggers concomitant autophagy and apoptosis in a rat chronic spinal cord compression model. Mol Med Rep 9(6): 2091-2096.
- Park Y, Liu C, Luo T, Dietrich WD, Bramlett H, et al. (2015) Chaperone-Mediated Autophagy after Traumatic Brain Injury. J Neurotrauma 32(19): 1449-1457.

 Mini Review
Mini Review