Abstract
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has become a Public Health Emergency of International Concern (PHEIC) causing mortality due to cytokine storm syndrome and multiorgan failure. Corticosteroids have shown considerable efficacy in studies conducted on COVID-19 patients but the relative efficacy and safety for its compassionate use remain unclear. This meta-analysis aimed to evaluate available evidence on the efficacy of corticosteroids in the management of COVID-19 patients at various stages of the disease. In this meta-analysis, we included seven trials, and their pooled analysis revealed COVID-19 patients in the treatment group (n=3334) was lower as compared to the control group (n=5585). The overall pooled analysis among COVID-19 patients showed corticosteroids could decrease mortality (OR = 0.587, CI = 0.36-0.95, and p-value 0.029). Corticosteroids had an insignificant effect on viral clearance. Findings suggest that corticosteroids are helpful in managing COVID-19 patients although further research is required.
Keywords: Coronavirus; SARS-Cov-2; COVID- 19; Treatment; Therapeutic Agents; Drugs
Abbreviations: COVID-19: Coronavirus disease 2019, PHEIC: Public Health Emergency of International Concern (PHEIC), OR: odds ratio, CI: confidence interval, SARS: Severe acute respiratory distress syndrome, MERS: middle east respiratory syndrome, RTPCR: real time polymerase chain reaction, MDs: mean differences, HR: Hazard Ratios, IV: intravenous, ICU: intensive care unit, COPD: Chronic obstructive pulmonary disease, ARDS: Acute Respiratory Distress Syndrome, CRP: C-reactive proteins
Background
Coronavirus disease-19 (COVID-19), a respiratory viral disease caused by SARS-COV-2, enveloped, and single-stranded coronavirus belongs to subgenus Sarbecovirus. The first case of COVID-19 turned up in Wuhan, a city of China. It spread rapidly and became the global epidemic affecting more than 213 countries across the globe [1]. According to the World Health Organization, the number of cases across the world reaches 23 million, and the number of death is 805,902 as of August 24, 2020 [2]. Some studies reported that most of the patient shows no symptoms at all or very mild symptoms and only 20 percent of patients develop respiratory problems and need hospitalization [3]. The morbidity of the patients is due to the progression of the respiratory infection to the hypoxemic respiratory failure and cytokines releasing syndrome, often requiring prolonged mechanical ventilation. The fatality rate reaches 26% in the United Kingdom and more than 37% in ventilation requiring patients [4]. According to available literature the corticosteroids were widely used in previous SARS pandemic in 2003-4 in China and Hong Kong, and this leads to the usage of corticosteroid in COVID-19 in clinical trials [3,4]. Previously COVID-19 has been treated with drugs from various classes like antivirals, immune-modulating therapies, and anti-inflammatory drugs, and corticosteroid is an attractive option because of its potent anti-inflammatory mode of action and its previous regular usage in other respiratory conditions like severe acute respiratory distress syndrome. [5] But the use of systemic corticosteroid treating COVID 19 disease still contend because one of the recent meta-analysis showed that patients treated without hydrocortisone show a low viral load in plasma and decreased viral shedding time than those treated with hydrocortisone [6].
According to some experts, corticosteroids increase viral shedding in lung damage and shock induced by a coronavirus and hence should not be used. [7] Various other trials showed promising results like recently in one of pre-published study, a randomized trial is conducted using a sample size of 11,303 patients in the different stage of COVID-19, show a reduction in 28 days mortality in the patients on oxygen therapy and mechanical ventilation using dexamethasone and their results lead to the global use of dexamethasone in COVID-19 patients [8]. Retrospective studies showed promising results of corticosteroid in SARS patients while in MERS patients, the usage of corticosteroids is not satisfactory, and patients require ventilation and renal replacement therapy. [9] In a nutshell, various trials and case studies showed a wide range of results. As the literature is rapidly expanding and continuously refilled every day, an updated meta-analysis featuring the latest literature is required. The aim of this systematic review and meta-analysis to assess the strength of evidence of usage of dexamethasone and other corticosteroids in the treatment of COVID-19. The study aims to obtain a single summary to quantify the results.
Materials and Methods
Search Strategies
In our study, we collected data by searching published articles in peer-reviewed journals by doing online search on article search engines like PubMed, Google Scholar, EMBASE, and Cochrane database from June 15th to July 31th, 2020. We searched articles by searching keywords “Coronavirus”, “SARS-CoV-2”, “Corticosteroids”, “Dexamethasone”, “treatment”, and “effectiveness”. We also searched these databases with individual drug names of corticosteroid family like, prednisolone, methylprednisolone, betamethasone, cortisone, and hydrocortisone. We also searched for the articles mentioned in the references of these articles.
Study Selection
We included randomized control trials and cohort studies investigating the effect of corticosteroid therapy among COVID-19 patients provided that: the medium of the reported article was English, the participants of the study were 18 years old or older, COVID-19 testing was done through RT-PCR, and analysis & outcome measures were given. We included the articles published in peer review journals from June 15th to July 31th 2020. The articles like editorials, perspectives, commentaries and short reviews were excluded.
Data Extractions
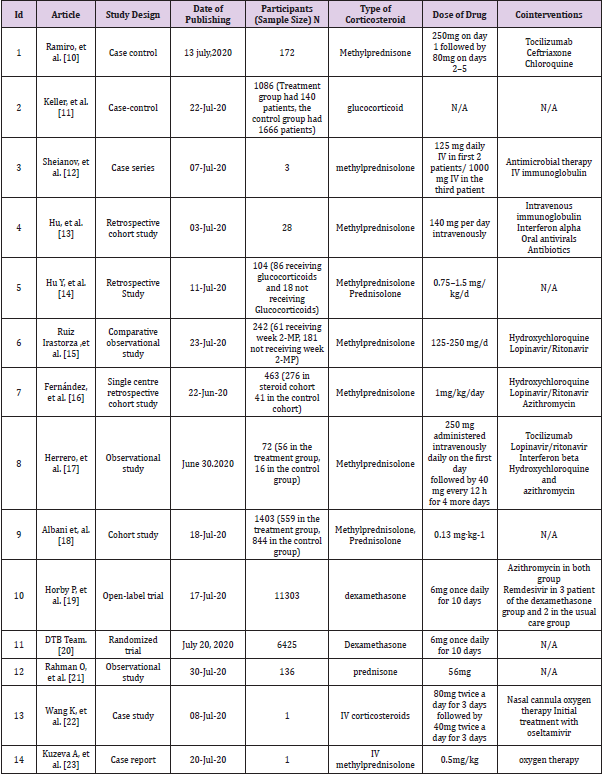
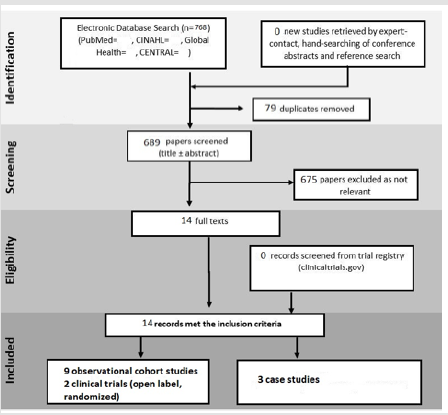
Two authors (SA and A) screened the rest of the articles independently. The articles on which two authors were of different views, whether to include them or not, the opinion of the third author (MASC) was obtained, and thus, the articles were screened out without any bias. The data we extracted and tabulated were the name of the articles, publication date, interventional drugs, number of participants, and any co-interventions used. Screening of the articles yielded a total number 14 articles following PRISMA (Preferred Reporting Items for Systematic Reviews and Meta- Analyses) guidelines, as shown in Figure 3.
Data Analysis
We conducted meta-analysis on the factors: effect of corticosteroid therapy on mortality, length of stay, ICU admission, adverse events, and risk of infection. Data analysis was conducted on RevMan 5.4. We analyzed pooled mean differences (MDs) and pooled adjusted Odds Ratios (OR), Hazard Ratios (HR), with 95% CIs , using the generic inverse-variance approach. The pooled analysis is shown graphically by forest plot. χ2 Cochran’s Q test was employed to assess the statistical heterogeneity of studies. p value of 0.05 less was considered significant. For assessment and graphical representation of the heterogenicity and bias, we plotted the funnel plot using individual studies.
Results
Search Results
Our database searches yielded 768 relevant articles. After removing 79 duplicated articles and screening titles and abstracts, 675 articles were excluded because of irrelevance. Full-text articles for 14 articles are assessed for eligibility, and 7 articles are included for final meta-analysis. Among 14 articles, 9 were observational cohort studies, 2 clinical trials (open label, randomized), and 3 were case series involving 8632 patients.
Study Characteristics
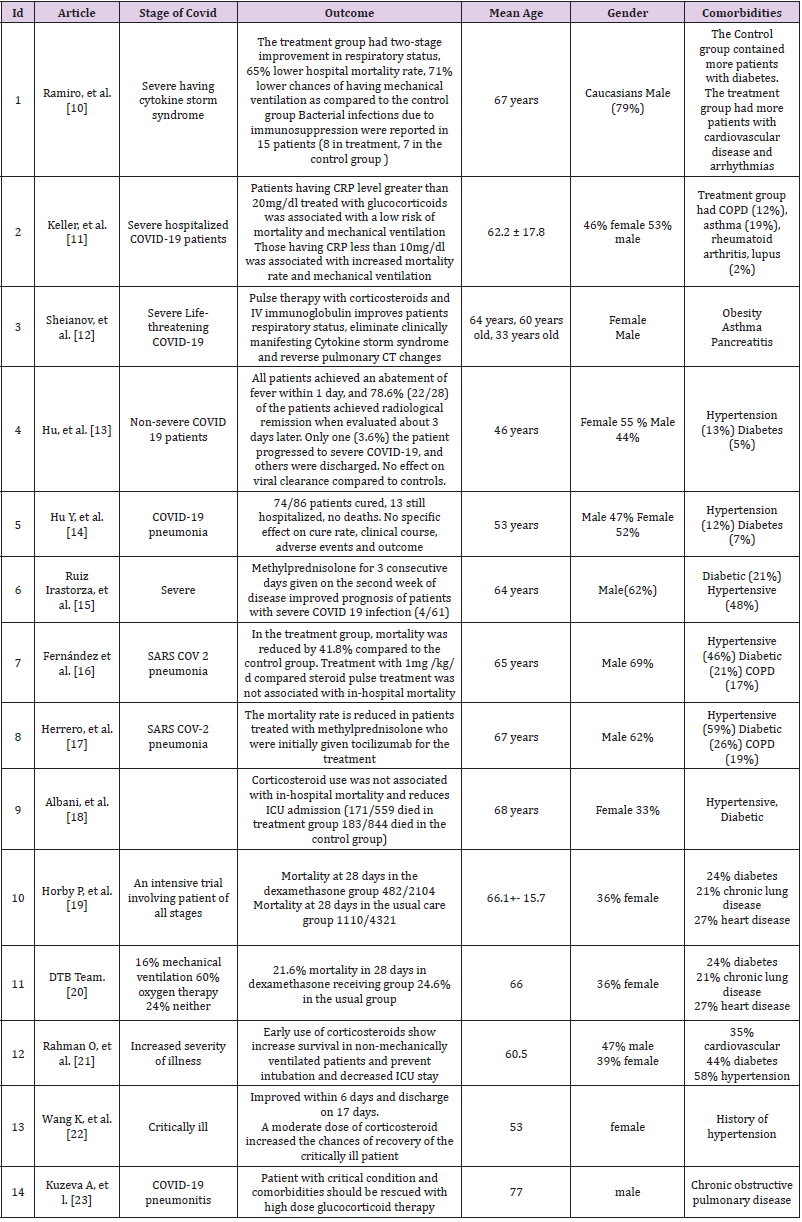
As 6 articles did not report relevant data on mortality, we reported outcomes on 7 studies (3334 patients receiving steroids and 5585 patients in the control group or not receiving steroids). The patient’s mean age in the selected studies was 61.93 year and 63.61% are males and 36.69% are females. Seven studies reported mortality. 9 studies showed participants receiving other cointerventions. Hypertension and diabetes were common in 11 studies. Corticosteroid most commonly used was methylprednisolone, but 2 studies included dexamethasone for treating COVID 19 patients. Seven articles included patients with severe SARS CoV-2 infection, 4 studies with COVID 19 pneumonia, one study included patients at all stages of COVID, and one study with non-severe patients. Among 14 articles, 13 specified dosing and duration with corticosteroid treatment.
Viral Clearance
Among the 14 studies included in the metanalysis, 3 of them discussed the differential outcomes of corticosteroid therapy on viral clearance in treatment and control groups. showed that in both treatment and control groups, the difference in the viral clearance was insignificant (p = 0.252), which means that the time from onset of illness to viral clearance had no significant difference between two groups. studied the effect of methylprednisolone treatment on time taken for viral clearance. The results were insignificant in this trial (p = 0.713). Collectively, corticosteroids show no significant effect on viral clearance.
Length of Hospital Stay/ ICU days
Several studies investigated the effect of corticosteroid therapy on the length of hospital stay. [10, 11] showed that the patients taking corticosteroids stayed admitted to the hospital for a longer time than the control group. The length of stay in the treatment group on average was 25 days, while the untreated group remained admitted on an average of, and these results are significant (p = 0.016). (Table 1) showed that the length of stay in the treatment group was less than that of the duration of hospital stay in the usual care group. Herrero et al. showed an insignificant relationship between a hospital stay and corticosteroid therapy (p = 0.091). in subgroup analysis, compared the timing of initial steroid dosing with ICU stay days when given less than 48 hours, mid between 48 hours and 7 days, and greater than 7 days after ICU. The association between use of corticosteroids and length of hospital stay was insignificant.
Mortality Rate
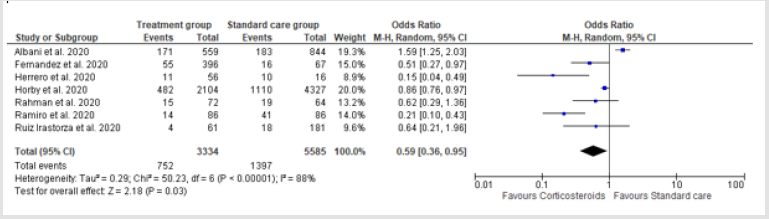
Out of 14 studies, 7 studies, i.e., Ramiro et al., Fernandez et al., Rahman et al., Horby et al., Ruiz Irastoza et al., Herrero et al., and Albani et al. investigated effect of corticosteroid therapy on mortality rates (p-value=0.029). The pooled analysis among COVID-19 patients revealed significant effect of corticosteroids on mortality. Mortality rate was lower in the corticosteroid treated group. (OR = 0.587, CI = 0.36-0.95, and p-value 0.029). There was an evidence of significant heterogeneity between trials, i.e., I2 = 88% and p-value = 0.000.
Adverse Events
Hue et al. reported the effect of corticosteroid on fasting blood glucose before and after the intervention. Corticosteroids have no significant effect on fasting blood glucose (p = 0.845). There was a trend towards bacterial infections in the group treated with corticosteroids. Two studies reported nosocomial infections associated with steroid administration. According to Ruiz Irastorza et al. non-pulse glucocorticoids have increased risk for infections (OR=4.72, 95% CI 1.9-11.8, p < 0.001) as compared to week 2 MP (OR=1.04, 95% CI 0.40-2.70, p=0.938). According to [12]., pulmonary embolism risk was significantly greater in the treatment group as compared to the control group (p = 0.0590). In [13]. bacterial infections are reported in 15 patients (8 in the treatment group vs 7 in the control group p = 0.787). Shieanov et al. discussed a report of three clinical cases in tocilizumab resistant COVID-19 patients and reported pulse therapy with glucocorticoids and IV immunoglobulins could help reverse respiratory failure and CT changes Figure 1.
Figure 1: Funnel plot for the assessment of publication bias among included studies. There is no evidence of publication bias.
ICU Admission
In Albani et al. 56 patients (11.5%) were admitted to ICU in patients exposed to corticosteroids on the ward vs 131 (14.4%) in the control group (unadjusted p = 0.15). In Herrero et al., 31 out of 56 patients treated with methylprednisolone were admitted to ICU as compared to 12/16 in non-methylprednisolone group (p = 0.158) Figure 2.
Publication Bias
In order to check the publication bias, we plotted studies included in a literature on a funnel plot. Resultantly, no publication bias was founded (Table 2).
Discussion
In this systematic review and meta-analysis of the effect of
corticosteroid treatment on COVID-19 patients, a pool of 14 studies
were included. Most of the studies showed the positive effect of
corticosteroid therapy on the mortality of the patients but no
significant effect on the viral clearance from the onset of illness. The
mean time of viral clearance in treated and non- treated patients
group was same. An increase hospital stay was observed in treated
group as compared to control group [13]. Corticosteroid, as evident
by a randomized trial study, decrease the “28-day mortality” [14]
on invasive mechanical ventilation requirement. So, we can say
that corticosteroids when administered in the COVID-19 patients
shown marked improvement in their condition. However, there is
an ambiguity about their effect on viral clearance and hospital stay
duration. In comparison with other systematic reviews and metaanalysis,
our study consists of a variety of studies with different study
designs including case-control, observational studies, randomized
and open trials, and even case studies, so it has a minimum risk
of bias and increased evidence level. Our meta-analysis includes
a recent constellation of literature on corticosteroids therapy and
COVID-19 patients in a large number of recent recovery trials.
Hence, it provides up-to-date and refined shreds of evidence.
In addition, all studies included in this meta-analysis provide
evidence only on corticosteroid therapy for COVID-19 patients while
some reported systematic reviews and meta-analysis, extrapolated,
results from corticosteroid effect on MERS, SARS and non-viral Acute
Respiratory Distress Syndrome (ARDS). With the high publication
rate of COVID-19 articles in the past few months, it is necessary to
include peer-reviewed articles as some of the most recent studies
concluded that corticosteroid is not safe for administration in
COVID-19 pneumonia with certain adverse reactions not included
in the previous meta-analysis. Most of the included studies have
large heterogeneity (the type of study, sample size, corticosteroid
used in the study, dose, duration, cointerventions, comorbidities,
and outcome measures, etc). Also, the reported meta-analysis
included studies completed in one area or countries like China, so
their results cannot be applied to the patients of other countries
but we collected the evidence from studies carried out in different
countries so, our results can be applied in every region of the world.
[15-17] The optimal time for administration of steroids for better
outcomes is the point of discussion in various reviews but none of
the studies answered the mystery. But our results from included
reports show that corticosteroid administration within 7 days
after onset of illness is associated with better recovery, improved
condition, and decrease ICU stay [18]. Methylprednisolone is the
most commonly used steroid in the included studies.
And its administration on 1st, 2nd, 5th and 7th day from
onset of illness show promising results. There are certain cointerventions
along with corticosteroids including (tocilizumab,
IV immunoglobulins and oseltamivir). The administration of
corticosteroid with tocilizumab shows more promising results
than corticosteroid alone. It reverse CT changes, decrease mortality
but shows an increased risk of bacterial infection [10,17]. Coadministration
of steroids with IV immunoglobulins in critical
patients improves the severity of the disease, reverses cytokine
syndrome, CT changes, and decreases mortality. Oseltamivir is
administrated in a few patients as initial patient care prior to the
administration of dexamethasone but no comment is given on
its outcomes. [19] According to one negative study, that reports
monotherapy with methylprednisolone having no effect on cure
rate, death rate, clinical course, and adverse effects [14]. The studies
included in this report have a variety of patients with different
stages of COVID-19, so results concluded from these studies can be
applied to any patient with any stage of disease not given by any
other published systematic reviews and meta-analysis.
Almost every patient included in these studies has one of the
comorbidities (diabetes, hypertension, heart diseases, chronic
obstructive pulmonary disease and pancreatitis, etc) so these
patients tend to progress to the more severe stage of disease.
Such patients should be rescued with a high dose of corticosteroid
therapy.[24] Hence, the conclusion drawn from these studies cannot
be applied to patients having mild symptoms and patients without
any comorbidities. One of the major findings not reported in the
previous analysis is the level of C-reactive protein (CRP) in plasma
and its association with corticosteroid therapy. Patients having
CRP levels greater than 20mg have a less need for mechanical
ventilation and a decreased mortality rate. While Patients having
CRP levels lower than 10mg tends to have the severe clinical course of the disease and increase the mortality rate. [20] The age of most
of the patients is in the range between 50- 80 years. So conclusions
drawn from these patients cannot be applied to younger patients.
COVID-19 patients faced duel problems. First, the patients develop a hyperinflammatory response against the virus in later stages that may cause pulmonary thrombosis and progress to acute respiratory syndrome and secondly, there is a need for viral clearance. Corticosteroid has an anti-inflammatory effect to cope with inflammatory response and cytokines release but not with viral shedding and hence, have certain adverse reactions. One of the adverse reactions reported by other meta-analysis is fasting hypoglycemia. But corticosteroids have an insignificant effect on fasting glucose levels [21]. The major side effect faced by patients on steroids, is an increased risk of the bacterial infections [22]. Nosocomial infections associated with steroid therapy are reported by two studies. Non-impulse glucocorticoids have more risks for infections. Pulmonary embolism is another adverse effect associated with corticosteroids. Control groups have low chances of development of pulmonary embolism than the treatment groups. [23] Tocilizumab resistant COVID-19, treated with corticosteroids and IV immunoglobulins have an increased risk of respiratory failure and severe CT changes. [12] Our systematic review and meta-analysis have certain limitations too. What is lacking is the specific indications of corticosteroid administration. We comment on only one laboratory marker CRP [12], other radiological and laboratory markers levels which can indicate the timing and dose of corticosteroid administrations should be investigated. Further researches on steroids administration on younger patients, patients with mild symptoms, and without comorbidities are needed which are lacking in all of the previous studies. More randomized trials and open-label trials are needed to investigate viral clearance and viral shedding, dose, indications, and optimal time for administration of corticosteroids. Most trials used co-administration of other drugs with corticosteroid so having little authenticity on the outcomes of monotherapy of corticosteroids.
In conclusion, combination therapy of corticosteroids with tocilizumab or IV immunoglobulins are associated with surprisingly better outcomes, decreased rate of mortality, decreased in hospital stay, reversal of cytokine storm syndrome, and CT changes. Certain adverse effects are associated with them. Bacterial infection is the major risk. Interventions should be made to cope with them. Because of the expanding knowledge and easy and over the counter availability of the steroids by healthcare systems, and the rapid spread of coronavirus across the globe, the area of corticosteroid research should be emphasized.
Conclusion
Recent evidence on the corticosteroids shows that the use of corticosteroids in various stages of coronavirus disease cause a significant effect on mortality and insignificant effect on viral clearance. Anti-inflammatory action of corticosteroids does not improve the time of viral clearance. The mean time for viral clearance in the affected patients did not vary much in the corticosteroid treated and the untreated group. Corticosteroids therapy shows varying effect on the length of hospital stay. Corticosteroids may increase or decrease the hospital stay durations. Corticosteroids, due to their immune suppressive effect, significantly increase the risk of bacterial infections. Corticosteroids improve respiratory symptoms, and often reverse the adverse CT findings. Corticosteroids therapy increases the risk of pulmonary embolism. Recent evidence shows a weak link between corticosteroid therapy and admission in Intensive Care Unit (ICU).
Availability of Data and Materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgment
Not applicable.
References
- Wang JN, Yang WX, Chen PW, Guo JB, Liu R, et al. (2020) The proportion and effect of corticosteroid therapy for patients with COVID-19 infection: a systematic review and meta-analysis.
- (2020) WHO Director-General's opening remarks at the media briefing on COVID-19 - 3 August 2020 [Internet]. Who.int. 2020 [cited 16 August 2020].
- Hasan SS, Capstick T, Ahmed R, Kow CS, Mazhar F, et al. (2020) Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta-analysis. Expert review of respiratory medicine 14(11).
- Group RC, Horby P, Lim WS, Emberson JR, Mafham M, et al. (2019) Dexamethasone in Hospitalized Patients with Covid-19-Preliminary Report. The New England journal of medicine.
- Rahman O, Trigonis RA, Craft MK, Kruer RM, Miller EM, et al. (2020) Corticosteroid Use in Severely Hypoxemic COVID-19 Patients: An Observational Cohort Analysis of Dosing Patterns and Outcomes in the Early Phase of the Pandemic. medRxiv. 2020 Jan 1.
- Li S, Hu Z, Song X (2020) High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2020 Jun 26.
- Wang K, Tan F, Zhou R, Liu D, Ni Z, et al. (2020) Therapeutic response to corticosteroids in a critically ill patient with COVID-19: A case report. Medicine 99(31): e21597.
- Hoekstra EM, Neumann KM, Boot PC, van Paassen J, Arbous SM, et al. (2020) Corticosteroid use in COVID-19 patients: A systematic review and meta-analysis on clinical outcomes.
- Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q, et al. (2020) The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. Journal of Infection 81(1): e13-e20.
- Ramiro S, Mostard RL, Magro-Checa C, van Dongen CM, Dormans T, et al. (2020) Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: results of the CHIC study. Annals of the rheumatic diseases 79(9): 1143-1151.
- Keller MJ, Kitsis EA, Arora S, Chen JT, Agarwal S, et al. (2020) Effect of Systemic Glucocorticoids on Mortality or Mechanical Ventilation in Patients With COVID-19. Journal of Hospital Medicine. 15(8): 489-493.
- Sheianov MV, Udalov YD, Ochkin SS, Bashkov AN, Samoilov AS, et al. (2020) Pulse Therapy With Corticosteroids and Intravenous Immunoglobulin in the Management of Severe Tocilizumab-Resistant COVID-19: A Report of Three Clinical Cases. Cureus 12(7): e9038.
- Hu Z, Lv Y, Xu C, Sun W, Chen W, et al. (2020) Clinical Use of Short-Course and Low-Dose Corticosteroids in Patients With Non-severe COVID-19 During Pneumonia Progression. Frontiers in public health. 8: 355.
- Hu Y, Wang T, Hu Z, Wang X, Zhang Z, et al. (2020) Clinical efficacy of glucocorticoid on the treatment of patients with COVID-19 pneumonia: A single-center experience. Biomedicine & Pharmacotherapy 130: 110529.
- Ruiz-Irastorza G, Pijoan JI, Bereciartua E, Dunder S, Dominguez J, et al. (2020) Second Week Methyl-Prednisolone Pulses Improve Prognosis In Patients With Severe Coronavirus Disease 2019 Pneumonia: An Observational Comparative Study Using Routine Care Data. medRxiv. 2020 Jan 1.
- Fernandez-Cruz A, Ruiz-Antoran B, Munoz-Gomez A, Sancho-Lopez A, Mills-Sanchez P, et al. (2020) Impact Of Glucocorticoid Treatment In Sars-Cov-2 Infection Mortality: A Retrospective Controlled Cohort Study. medRxiv. 2020 Jan 1.
- Sanz Herrero F, Puchades Gimeno F, Ortega García P, Ferrer Gómez C, Ocete Mochón, et al. (2020) Methylprednisolone added to tocilizumab reduces mortality in SARS‐CoV‐2 pneumonia: An observational study. Journal of internal medicine. 2020 Jul 17.
- Albani F, Fusina F, Granato E, Capotosto C, Ceracchi C, et al. (2020) Effect of corticosteroid treatment on 1376 hospitalized COVID-19 patients. A cohort study. medRxiv. 2020 Jan 1.
- Horby P, Lim WS, Emberson J, Mafham M, Bell J, et al. (2020) Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. MedRxiv. 2020 Jan 1.
- Team DT. Dexamethasone for COVID-19: preliminary findings.
- Rahman O, Trigonis RA, Craft MK, Kruer RM, Miller EM, et al. (2020) Corticosteroid Use in Severely Hypoxemic COVID-19 Patients: An Observational Cohort Analysis of Dosing Patterns and Outcomes in the Early Phase of the Pandemic. medRxiv. 2020 Jan 1.
- Wang K, Tan F, Zhou R, Liu D, Ni Z, et al. (2020) Therapeutic response to corticosteroids in a critically ill patient with COVID-19: A case report. Medicine 99(31): e21597.
- Kuzeva A, Dost S, Lams B, Agarwal S, Furmedge DS, et al. (2020) Time-critical administration of corticosteroid rescue therapy for COVID-19 pneumonitis in a ward-based patient with chronic obstructive pulmonary disease. British Journal of Hospital Medicine 81(27): 1-4.

 Research Article
Research Article