Abstract
The Nemesis – SARS-CoV-2 Pandemic: Leaving in its wake millions of infections, accompanied by an immense magnitude of morbidity and multitude of mortality, and an unfathomable economic toll, the COVID-19 pandemic has led to a global impact. A vaccine is urgently needed to prevent the disease, thwart the complications and avert deaths resulting from transmission of the infection.
The Hubris – Vaccine Development: While most of the platforms of vaccine candidates have focused on the spike (S) protein and its variants as the primary antigen of COVID 19 infection, various techniques involved include nucleic acid technologies (RNA and DNA), non-replicating viral vectors, peptides, recombinant proteins, live attenuated viruses, and inactivated viruses. There are novel vaccine technologies being developed for COVID 19 using next-generation strategies for precision and flexibility for antigen manipulation on SARS-CoV-2 infection mechanisms.
The Elpis - Updates and Prospects: There were nine different technology platforms under research and development to create an effective vaccine against COVID 19. Although there are no licensed vaccines against COVID-19 yet, there are various potential vaccine candidates under development and advanced clinical trials. Out of them, few having undergone phase III clinical trials, may soon be available for use among the highrisk groups following emergency use authorization.
Conclusion – Hopes and Concerns: The hope of benefiting from the vaccine to the extent that it may be the only way to tide over and control the COVID-19 pandemic, is accompanied by the likely fear of adverse effects and opposition in public for COVID-19 vaccination, including the vaccine hesitancy. Further, there is concern among scientific circles that vaccine may have opposite of the desired effect by causing antibodydependent disease enhancement.
Keywords: COVID-19 Vaccines; Genomic Sequencing; Coronavac; Covaxin; EpiVac- Corona; Moderna’s vaccine; Oxford-Astra- Zeneca COVID-19 Vaccine; Pfizer-BioNTech COVID-19 Vaccine; Sputnik V; Vaccine technology platforms; Vaccine hesitancy
Introduction
Curbing the Infection
Leaving in its wake millions of infections, accompanied by an immense magnitude of morbidity and multitude of mortality, and an unfathomable economic toll, the COVID-19 pandemic has led to a global impact [1]. The disease has seriously affected the vulnerable groups in the society including those 65 years of age or older, persons with underlying conditions, and the economically deprived population. It is feared that more of devastation is likely to be witnessed in form of serious post-COVID-19 complications in the survivors especially of the serious illness. A vaccine is thus urgently needed to prevent the disease, thwart the complications and avert deaths resulting from transmission of the infection [2]. Although there are no licensed vaccines against COVID-19 yet, there are various potential vaccine candidates based on a variety of platforms including lipid nanoparticle mRNA, DNA, adjuvanted protein, inactivated virus particles, and non-replicating viral vectors are in various phases of clinical trials, including 11 vaccine candidates in phase 3 trials, followed by over a hundred vaccine candidates in preclinical testing [3]. The safety and immunogenicity data relating the vaccines in this context are important. The clinical significance of SARS-CoV-2 binding and neutralizing antibody titres and their ability to predict efficacy is needed to be evaluated and confirmed. Though the immune correlates of protection against SARS-CoV-2 are yet to be determined, the neutralising antibodies are thought to be associated with protection based on results from studies in COVID-19 non-human primate challenge models inferring that neutralising antibody response is correlated with protection [4]. These findings have led to the use of neutralisation assays to assess immune responses in recent human COVID-19 vaccine trials [5].
The Nemesis: SARS-CoV-2 Virus Invasion and the Pandemic
The Virus and the Disease
During December 2019, an outbreak of apparently viral pneumonia was reported in the city of Wuhan, in Hubei province in China. Soon the disease spread to other parts of China and several countries to become a pandemic. By 9 January 2020, it was established that the disease was caused by a novel coronavirus, 2019-nCoV or SARS-CoV-2 and was named COVID-19 [6]. Later, during the first half of January 2020, the Chinese researchers shared the genome sequence of the virus, followed by identification of the same by the Mutualized Platform for Microbiology (P2M), Pasteur Institute, Paris on 29 January 2020 from the samples taken from the initial suspected patients in France [7]. Following the sequencing of SARS-CoV-2 genome, an international response was triggered to develop a prophylactic vaccine to provide acquired immunity against COID-19. By April 2020, almost 80 companies and institutes in 19 countries were working on the vaccine for COVID-19. Initially it was said, including by WHO in February 2020, that it did not expect a vaccine for the disease to become available in less than 18 months. Later, it has been claimed by researchers that with the help of genetic Engineering, the COVID-19 Vaccine can possibly be made in months rather than years [8].
Various vaccines under development to combat the COVID-19 have been modelled on the original strain, which was common among hCoV-19 genetic sequences published during the initial months of the course of the disease pandemic. Understanding the evolution and mutations of SARS-CoV-2 during the COVID-19 pandemic is imperative for disease control and prevention through the vaccine programme. A spike protein mutation D614G has emerged supplanting aspartic acid (D) in the 614th position of the amino acid with glycine (G), hence the change known as D614G. The D614G mutation has supposedly enhanced viral replication in human airway tissues, enhanced viral survival in the upper airway of infected hamsters, and increased susceptibility to neutralization. It appears that the mutation may have increased the infectivity of the virus [9]. The work by Plante et al underlines the importance of this mutation in viral spread, vaccine efficacy, and antibody therapy [10]. It has been pointed out, the vaccines against COVID-19 are hoped to work against new G-strain, as well [11]. Further, the study involving Hamsters concluded that the D614G mutation may not reduce the ability of vaccines in clinical settings to protect against COVID-19 and the neutralizing antibodies are to be assessed against the circulating variant of the virus before clinical development.
SARS-CoV-2 Genomic Sequencing
The genome of SARS-CoV-2 is comprised of a single-stranded positive-sense RNA. It is composed of 13–15 (12 functional) Open Reading Frames (ORFs) containing ~30,000 nucleotides and contains 38% of the guanine-cytosine (GC) content and 11 proteincoding genes, with 12 expressed proteins [12]. The ORFs are arranged as replicase and protease (1a–1b) and major S, E, M, and N proteins. These gene products play important roles in viral entry, fusion, and survival in host cells. Basically, the genomic sequencing is a technique to interpret genetic information found within the virus. So far, there are over 1,000 COVID-19 genomes published worldwide [13]. The genomic sequencing helps in understanding when and where the version of the virus originated and how the virus is evolving. Sequencing the genome of SARS-CoV-2 virus also helps in understanding the disease transmission kinetics, its spread in population groups and planning and evaluating the containment efforts. In addition, it helps to track the viral mutations as the disease spreads. In general, the viruses circulating locally have small genetic changes compared to the ones that are circulating elsewhere. Thus, the genomic sequence can be used to estimate the infected population size and how the virus is spreading. Further, understanding of the genomic structure of the virus helps in developing drugs and vaccines for therapy as well as prophylaxis of COVID-19.
The Hubris: COVID-19 Vaccine Development Programmes
COVID-19 Vaccine Platforms
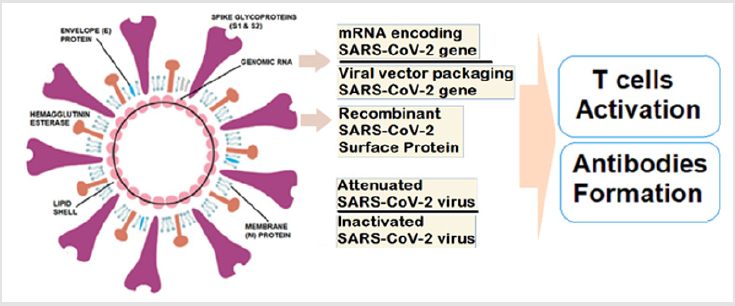
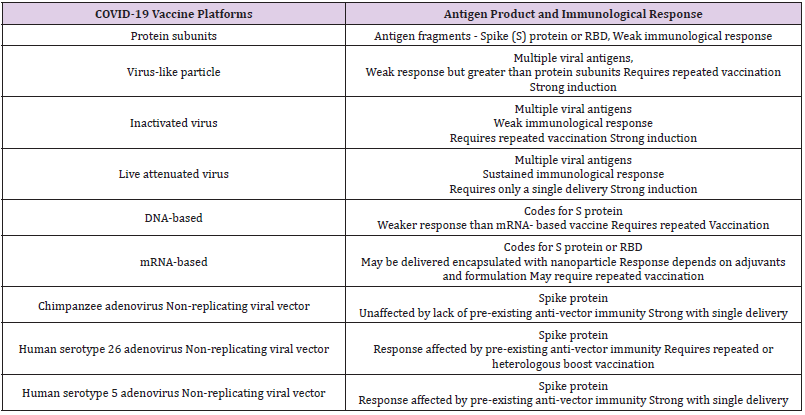
While most of the platforms of vaccine candidates have focused on the spike (S) protein and its variants as the primary antigen of COVID 19 infection, various techniques involved include nucleic acid technologies (RNA and DNA), non-replicating viral vectors, peptides, recombinant proteins, live attenuated viruses, and inactivated viruses [14]. The main protein, S protein to boost the immune system can be given as a vaccine in many different forms such as inactivated (dead) virus, as expressed protein, in a DNA or RNA vector that will lead the cells to make this protein and stimulate to make antibodies and activate T cells to control the viral infection or eliminate the infected cells to reduce disease severity and complications (Figure 1). As reported during September 2020, there were nine different technology platforms (Table 1) under research and development to create an effective vaccine against COVID 19 [15]. There are novel vaccine technologies being developed for COVID 19 using next-generation strategies for precision and flexibility for antigen manipulation on COVID 19 infection mechanisms [16].
Vaccine Development Stages and Clinical Trials
Vaccine development stages include
a) Exploratory or Preclinical Phase - Planning and designing
a candidate vaccine.
b) Phase I trials test primarily for safety and preliminary
dosing in a few dozen healthy subjects.
c) Phase II trials – following success in Phase I – evaluate
immunogenicity, dose levels and adverse effects of the
candidate vaccine, typically in hundreds of people. The phase I
and II consist of randomized and placebo-controlled trials.
d) Phase III trials typically involve several participants at
multiple sites including a control group. It aims to establish the
effectiveness of the vaccine to prevent the disease as well as
monitoring for the adverse effects at the optimal dose.
Currently, there are more than a hundred COVID-19 vaccine candidates under development, with several of them already in the human trial phase [17]. The WHO is working in collaboration with scientists, business groups, and various health organizations through the Access to COVID-19 Tools (ACT) Accelerator to streamline and speed up the effort. The WHO through the COVAX, which is one of three pillars of the ACT Accelerator, is bringing together governments, global health organizations, manufacturers, scientists, private sector, civil society and philanthropy, to provide equitable access to COVID-19 diagnostics, treatments and vaccines to protect people in all countries. In a statement issued by the International Coalition of Medicines Regulatory Authorities (ICMRA) and the WHO on 6 November 2020, it was endorsed that ICMRA and WHO are committed to ensure that people in various countries have access to safe and effective health products against COVID-19 as early as possible, while the scientific standards for the evaluation and safety monitoring of treatments and vaccines are rigorously maintained [18]. Simultaneously, they are working to ensure to reduce the risks associated with unproven treatments, and potentially fraudulent and false claims.
Enrolment and Plan of Clinical Trials
The human challenge studies or controlled human infection trials relating to vaccine development, involve an intentional exposure of the participants or test subjects to the vaccine products following preliminary proof of its safety and efficacy in laboratory animals and healthy humans. In this context, the fast-tracking for clinical trials for a COVID 19 vaccine involves compressing the clinical trial period of Phase II and Phase III trials from years to few months. Such challenge studies have been done earlier involving diseases like common flue, typhoid fever, cholera, and malaria which were less deadly than COVID 19. There is fear that fasttracking for clinical trials and bypassing typical Phase III research, providing for the accelerated path to license a COVID 19 vaccine may be disastrous and may expose the participants to dangers beyond those considered the anticipated potential side effects. The young adult volunteers are deliberately infected with COVID 19 in a challenge trial conducted and once an infection dose of COVID 19 is identified, the candidate COVID-19 vaccine is tested for effectiveness in preventing infection. Following the challenge, the participants are closely monitored. Although challenge studies are potentially hazardous for the participants, they are the only way to rapidly produce a vaccine that can be able to prevent the disease in estimated millions of human-beings worldwide and avert significant morbidity and mortality from COVID 19 infection [19]. The clinical COVID 19 challenge studies in healthy people are conducted as per the WHO Guidance Document including scientific and ethical evaluation, public consultation and coordination, selection and informed consent of the participants, and monitoring by independent experts.
The Efficacy of the COVID-19 Vaccines
The effectiveness of a new vaccine is defined by its efficacy [20]. The minimal efficacy limit set by WHO is 50 percent. Whereas an efficacy of less than 60 percent may fail to achieve herd immunity. There are other determinants for the efficacy and factors like genetics, health status (underlying disease, nutrition, pregnancy, and sensitivities or allergies), immune competence, age, obesity, and environmental factors, which may affect the susceptibility to infection and severity of the disease, and response to a vaccine. Further, the viral mutations altering its structure may have impact on the vaccine efficacy [21].
The Elpis: Vaccine Updates and Future Scenario for COVID-19
The Vaccines in Advanced Stages of Clinical trials
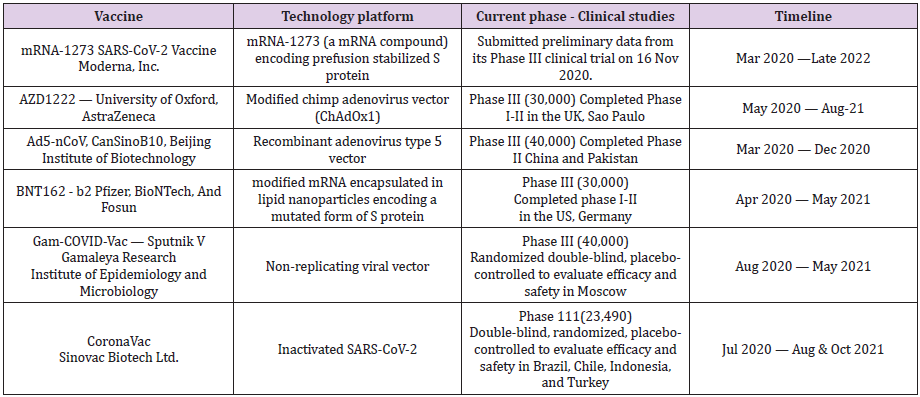
Out of various COVID-19 vaccine candidates in preclinical and clinical trials, only some are in advanced stages of clinical trials including 11 in phase 3 trials and few vaccine candidates are due for emergency use authorization (Table 2).
Moderna mRNA-1273 Vaccine : The mRNA-1273, the Moderna’s mRNA vaccine candidate against the SARS-CoV-2 virus, encodes for a prefusion stabilized form of the Spike (S) protein. Based on data from the results of the Phase 1 study, the dose of 100 mcg is generally well-tolerated across various age groups. Further in the older adults, it has been shown to produce the virus-neutralising antibodies at levels similar to that in the younger subjects [22]. Moderna has revealed no serious safety concerns. An independent board that conducted the interim analysis of the vaccine trials found side effects such as fatigue in 9.7 per cent of participants, muscle pain in 8.9 per cent, joint pain in 5.2 per cent, and headache in 4.5 per cent. More than 30,000 participants at 100 clinical research sites in the United States are participating in the study, which launched on July 27, 2020, after results from earlier stage clinical testing indicated that the vaccine candidate is well-tolerated and immunogenic. Recognizing the disproportionate impact of the epidemic on minority populations, the study has included 37 per cent of the trial volunteers from racial and ethnic minorities.
The vaccine has been co-developed by the Cambridge, Massachusetts-based biotechnology company Moderna, Inc., and the National Institute of Allergy and Infectious Diseases, a part of the National Institutes of Health. It combines Modern’s mRNA (messenger RNA) delivery platform with the stabilized SARS-CoV-2 spike immunogen (S-2P) developed by NIAID scientists. The vaccine will be manufactured at Visp, Switzerland by its partner Lonza Group, to produce the first doses in December of 2020. Another Lonza’s site at Portsmouth, New Hampshire, aims to produce it exclusively for the U.S.A. Recently, on 16 November 2020, Moderna, Inc. announced that the independent, NIH-appointed Data Safety Monitoring Board for the Phase 3 study of mRNA-1273, has confirmed that Moderna vaccine trial has met the statistical criteria pre-specified in the study protocol for efficacy, with the vaccine having efficacy of 94.5%. Later, on the following day, it announced that the European Medicines Agency human medicines committee has started a review of the vaccine, following the confirmation of eligibility of mRNA-1273 for submission on October 14, 2020.
Pfizer-BioNTech COVID-19 Vaccine: The mRNA-based COVID-19 vaccine candidate, BNT162-b2, developed by Pfizer and its German partner, BioNTech SE, has shown 95% effectivity. According to Pfizer data, of the 170 volunteers who contracted Covid-19 during Phase III trials involving over 43,000 people, 162 had received a placebo and only eight received the two-dose vaccine, indicating 95% efficacy of the vaccine. Further, Pfizer has claimed that the vaccine has consistent efficacy across different ages and ethnicities, and its efficacy in adults over 65 years, who are at particular risk from the virus, is over 94%. As per the data, the vaccine is well-tolerated and has mild to moderate side effects. About 2% volunteers in the study complained of headache, whereas 2% and 3.7% suffered with fatigue following the first dose and second dose, respectively. Further, there is safety data on about 100 children of 12-15 years of age and about 45% of US trial participants enrolled were 56-85 years old.
Pfizer Inc. and BioNTech SE have applied to US health regulators
for emergency use authorization (EUA) of its Covid-19 vaccine.
The Pfizer-BioNTech COVID-19 Vaccine is expected to receive an
EUA approval for its vaccine in the second half of December 2020.
Meanwhile, as on 2 December 2020, Medicines and Healthcare
products Regulatory Agency (MHRA) has approved the Pfizer/ BioNTech COVID-19 vaccine for widespread use in the UK.
Both the Moderna’s and Pfizer’s Covid-19 vaccines, rely on a
technology called messenger RNA, which is being used for the first
time to develop a vaccine. The technology is designed to tweak the
host cells to make certain proteins for immunological response.
These vaccines have specific cold chain and handling requirements.
Minus 70 degrees Celsius (-94°F) is required by the Pfizer vaccine,
whereas for Modern’s vaccine it is Minus 20 degrees Celsius. The
cold chain requirement, especially for the Pfizer’s vaccine can be
an obstacle for most Asian countries where high environmental
temperature is compounded by poor infrastructure. After reaching
a vaccination center, the vaccine is to be thawed and used within
five days.
AstraZeneca-Oxford COVID-19 Vaccine: The AstraZeneca- Oxford AZD1222 or ChAdOx1 vaccine triggers a robust immune response in adults aged 56-69 and over 70, as per the results of Phase II clinical trials. Further, it appears to be well tolerated across all age groups. As per the data, the immunogenicity was similar across age groups after a boost vaccination. These findings are encouraging especially in context of the older individuals, who carry a disproportionate risk of severe COVID-19 and a poor immunogenicity to vaccines because of immunosenescence. Immunization with ChAdOx1 nCoV-19 has shown to result in development of neutralizing antibodies against SARS-CoV-2 in almost 100% of participants including older adults without severe comorbidities, with higher levels in boosted compared with nonboosted groups [23]. The adverse reactions to the vaccine are mild, with the most common effects being injection-site pain and tenderness, feverishness, fatigue, headache, and myalgias. In India, the Phase III trials of the Oxford vaccine (named ‘Covishield’ in India) are being conducted by the Serum Institute of India. Recently on 20 Nov 2020, it was announced that the vaccine is planned to be tested on a limited basis by administering it to the frontline workers and the elderly. The vaccine is likely to be available for healthcare workers and elderly people by around February 2021 and by April for the general public. The Serum Institute of India has claimed that its vaccine has 90% efficacy.
Chinese COVID-19 Vaccine: The ‘Coronavac’, the Sinovac Biotech Ltd.’s COVID-19 vaccine, is a two-dose vaccine. It is under clinical trial involving 9,000 volunteers at Butantan Institute, São Paulo, Brazil. Recently, the trial was halted on 10 November, following a serious adverse event by the Brazil Health Agency. The vaccine is also in the final Phase III trials at University Research Hospital in Kocaeli, Turkey. The vaccine has been offered by Chinese city for emergency use 17 Oct 2020 onwards. Another Chinese COVID-19 vaccine, with Ad5-nCoV (Recombinant adenovirus type 5 vector) technology platform, named ‘CanSinoBIO’ is developed by the Beijing Institute of Biotechnology and under Phase III trials involving 40,000 volunteers in China and Pakistan.
Russian COVID-19 Vaccine: The Russian COVID-19 vaccine, named ‘EpiVacCorona’ is being developed by Vector State Research Center of Virology and Biotechnology. On 16 Oct 2020, Russia has announced completion of successful clinical trials of EpiVacCorona. Another Russian COVID-19 vaccine named ‘Gam-COVID-Vac’, also known as ‘Sputnik V’, is based on the non-replicating viral vector technology platform and being developed by the Gamaleya National Research Center for Epidemiology and Microbiology and the Russian Direct Investment Fund (RDIF). Sputnik V vaccine is claimed to be 92% effective at protecting people from COVID-19 according to the interim trial results. The vaccine is to be kept at a temperature of -20 to -70 degrees Celsius. The Russian vaccine, Sputnik V, is under mid- to late-stage clinical trials by Hyderabadbased Dr Reddy’s Laboratories in India.
Other COVID-19 Vaccines: In India, trials are underway for ‘Covaxin’, an indigenous COVID-19 vaccine being developed by the Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and National Institute of Virology (NIV), Pune. Covaxin uses a part of inactivated SARS-CoV-2 virus to provoke the immune response. The vaccination schedule consists of two doses of Covaxin for each study participant, administered via intramuscular injection 28 days apart. The Phase III of the human clinical trial of Covaxin is currently underway at All India Institute of Medical Sciences, New Delhi. As stated in a latest communique by Bharat Biotech on 23 Nov 2020, Covaxin is expected to be 60% efficacious, the vaccine can be stored at temperatures between 2°C and 8°C, economical at a projected cost between Rs 500 and Rs 600 per dose for the general public and could be ready for rollout by next year, in June 2021.
Another COVID-19 vaccine ‘ZyCOV-D’, developed by the Indian pharma giant, Zydus Cadila, using the DNA based platform using non-replicating and non-integrating plasmid carrying the gene of interest, is currently under phase II of human trials. According to the data from the preclinical stage, the vaccine was found to be immunogenic in multiple animal species and the antibodies produced were able to completely neutralize the wild type virus. In the Phase I clinical trials, the vaccine candidate was found to be safe and well tolerated. The vaccine is expected to be available by March 2021. The British researchers are also studying inhaled versions of COVID-19 vaccine candidates to see if they can deliver a localised immune response in the respiratory tract. An alternative to the common injection in the arm, the spray vaccine is supposed to trigger specific immune responses in airways by mimicking the natural infection of a respiratory virus. China is also set to start trial of nasal spray COVID-19 vaccine. The researchers working on inhaled vaccine plan to utilize some of the unique cellular features of the lungs, nose, and throat.
COVID-19 Vaccination - Other Issues
The Fast Tracks for COVID-19 Vaccines: Because of the urgency created by the COVID-19 pandemic, the development of various vaccines is on a fast track. Classically, for a vaccine the preclinical stage is about 18 to 30 months, followed by the phase I, II and III, each of them lasting for about two and half years, and the approval followed by production of the vaccine taking a period of another one to two years. For the COVID-19 vaccines being developed urgently, the preclinical stage is short one, followed by phase I and II each compressed to duration as short as 6 months and the phase III shortened to zero month, and the COVID-19 vaccine is foreseen to get an approval for emergency use and start its production simultaneously. The urgency and the haste are likely to involve errors at multiple stages and carry a potential scope for disaster.
Timeline for the COVID-19 Vaccination: It is being envisaged that following the emergency authorization for use by US Food and Drug Administration, European Medicines Agency (EMA) or other governing body in a country concerned, the vaccine will initially be offered to people at high risk for the disease and healthcare workers (Dec 2020 -March 2021), followed by those at risk (March 2021 or later), before being available to the general population (July 2021 or later). It is thought that a target to vaccinate 75 percent population is likely to attain localized herd immunity.
Opposition to the COVID-19 Vaccines: The hope of benefiting from the vaccine to the extent that the vaccine may be the only way to tide over and control the COVID-19 pandemic, is accompanied by the likely opposition in public for COVID-19 vaccination, including the vaccine hesitancy. As apparent from various surveys, some people are understandably concerned that the speed of both scientific review and vaccine regulation could compromise safety, despite vaccine developers’ and regulators’ assurances to the contrary. Vaccine distribution poses another formidable challenge. It is also accompanied by issues such as its cost and who will be paying for it. In a Medscape reader poll involving 308 UK physicians, it was found that 4 in 10 doctors would not have a COVID-19 vaccine as soon as one is approved by the Medicines and Healthcare products Regulatory Agency in the country. About 56% cited safety concerns, 27% would rather wait, 7% mentioned personal health reasons, and 14% had other reasons. Overall, 59% said vaccination for healthcare staff should not be compulsory [24]. With the growing number of people who oppose the vaccination, their attitudes have also changed over last few months. The polling by Kantar of 1000 people carried out recently on 10-11 November, found that 76% of people in Britain would like to take a vaccine for COVID-19, but the score has fallen since June 2020.
In another European survey, 73.9% participants were willing to get vaccinated against COVID-19 if a vaccine would be available; 18.9% of respondents stated that they were not sure, and 7.2% stated that they do not want to get vaccinated [25]. The common reasons for opposing COVID-19 vaccination, are based on the belief that the vaccine may not be safe (24%), the concern about the side effects (21%), considering the COVID-19 infection not dangerous (14%), rejecting vaccinations as a general principle (11%), and some (8%) not willing to meddle with the course of the Nature [26].
The COVID-19 Vaccine Nationalism
The Covid-19 pandemic has triggered a global race for development of its vaccine. Further, there has evolved a competition among various countries to ensure the COVID-19 vaccine availability to their citizens. The wealthy governments have invested in vaccine candidates and have made bilateral agreements with developers, resulting in the vaccine doses being reserved. With over 1.5 billion potential dose purchases and 1 billion confirmed dose purchases, the US alone has signed up for more than 2.6 billion doses. This is followed by the European Union’s 1.2 billion doses and another 750 million potential purchase. India has confirmed dose purchases exceeding 1.5 billion, ranking third in terms of the number of Covid-19 vaccine doses committed to procure. Thus, there are already more than 8 billion doses of Covid-19 vaccine currently reserved due to advance market commitments before a clearly confirmed outcome of the effectiveness of a COVID-19 vaccine is released. These factors may hinder the delivery of health innovations to several lower-income countries and potentially leave their populations vulnerable to COVID-19. The advanced deals made by high-income countries as well as middle-income countries have led to a fear that this may create a challenge for equitable global distribution of coronavirus vaccines [27]. In response to the vaccine nationalism, there has been the creation of the COVAX Facility led by the WHO, which is an international partnership aiming to financially support leading vaccine candidates and ensure access to vaccines for lower-income countries. Seventy-nine higherincome countries are COVAX members. Their governments will help support 92 lower-income countries for affording COVID-19 vaccines.
The WHO Stand on COVID-19 Vaccination
The populations at greatest risk of serious COVID-19 include people with coexisting health conditions and older adults. A safe and effective vaccine will be an important tool in controlling the global COVID-19 pandemic and the success rate of a vaccine to be introduced for use, as per the WHO recommendations, should show the disease risk reduction by at least 50 per cent. It is estimated about 70% of people must be inoculated to end the pandemic, and Asia alone is home to more than 4.6 billion - or three-fifths of the global population. Further, the Director General of WHO has cautioned that the COVID-19 vaccines alone will not be enough to stop pandemic and that the vaccines should complement the other tools such as universal masking and social distance, not replace them.
Vaccine related Injury and Compensation
As early as 2005, the International Federation of Pharmaceutical Manufacturers and Associations demanded that manufacturers be granted protection from lawsuits associated with vaccine-related adverse events if they were going to participate in pandemic responses. Following which, in the United States, the Public Readiness and Emergency Preparedness Act was passed in the background of clinical trials of avian influenza vaccine, providing manufacturers immunity from lawsuits related to injuries caused by vaccines, with narrow exceptions, in the event of a declared public health emergency. In the current situation the same kind of immunity is being claimed by the vaccine manufacturers [28]. About 25 countries have no-fault vaccine-injury compensation systems for routine immunizations and required changes could be made to policies related to funding, proving injury, and distributing compensation. Other countries need to agree to appropriately provide legal immunity and indemnify the WHO, donors, manufacturers, and health care workers who vaccinate people. Simultaneously, there is required a mechanism for efficiently handling a high volume of claims from throughout the world. To meet this, it is being explored that the COVAX Facility may establish a procedure for compensating people who may suffer from severe adverse events related to the vaccination. Further, the international body for compensation based at the COVAX Facility may be practicable solution to facilitate the procurement of COVID-19 vaccines while ensuring that vulnerable people are able to seek compensation for injuries, and it could as well set a precedent for future vaccination campaigns.
Conclusion
Immunological Response to the Vaccines
Understanding immune memory to SARS-CoV-2 is critical for improving diagnostics and vaccines, and for assessing the likely future course of the pandemic. The various components of immune memory of SARS-CoV-2 tend to persist for about 6 months [29]. The S-specific memory B cells are more abundant at 6 months than at 1 month. Whereas the SARS-CoV-2-specific CD4+ T cells and CD8+ T cells decline with a half-life of 3-5 months. The neutralizing antibodies appear to be the only component of immune response that can provide protection from the infection. The spike IgG titres show a modest decline in 6 to 8 months. But the magnitude of the antibody response against SARS-CoV-2 is highly heterogenous between individuals. Immunization studies in non-human primates have indicated that circulating neutralization titres of 200 or more may provide sterilizing immunity. Further, the presence of sub-sterilizing neutralizing antibody titres at the time of exposure to infection may blunt the size of the initial infection and may contribute to limit the disease severity.
Efficacy Vs Exaggerated Immune Reactions
The confirmation of the correlation between antibody titres and protection against Covid-19 can only be possible through a large clinical efficacy study. In the meantime, the assays for measuring antibody may fill the gap but their validity needs to be ascertained. There is an uncertainty relating to the expected efficacy. It is being projected, depending on the profiles observed for other viral vaccines, that the vaccine’s efficacy against severe COVID-19 may be higher than efficacy against mild disease. But there is another aspect of the immunological response to the vaccine. Although the antibody production by a potential vaccine is intended to neutralize the COVID 19 infection, it is feared that the vaccine may have an opposite effect by causing Antibody-Dependent Disease Enhancement (ADE), which might trigger the cytokine storm in case the person is infected by the virus in future, after the vaccination [30]. The technology used for vaccine, its dose, timing of repeat vaccinations for the possible recurrence of COVID 19 infection, and elderly age are factors related to the risk and extent of ADE.
The Vaccine Hesitancy and Concerns
The rapid development and urgency of producing a vaccine for the COVID 19 in view of the raging pandemic may increase the risks and failure rate of delivering a safe and effective vaccine. There are indications that the potential success rate may be only 10% for various COVID-19 vaccine candidates under development [31]. On the other hand, at least 10 per cent of the people in different surveys perceive the COVID-19 vaccines as unsafe or unnecessary and consider refusing the vaccination. This public perception has been called vaccine hesitancy [32]. Such behaviour can increase the risk of further viral spread that could lead to future COVID 19 outbreaks. As per a survey in the United States about 67% or 80% of people would accept a new vaccination against COVID 19, with wide disparity relating to education level, employment status, and racial and geographical background [33]. Similar and comparable findings have been documented in a study from the United Kingdom [34].
References
- Jackson LA, Anderson EJ, Rouphael NG (2020) An mRNA Vaccine against SARS-CoV-2-Preliminary Report. N Engl J Med 383(20): 1920-1931.
- Heaton PM (2020) The Covid-19 Vaccine-Development Multiverse. N Engl J Med 383(20): 1986-1988.
- (2020) World Health Organization, Geneva. Draft landscape of COVID-19 vaccine candidates.
- Corbett KS, Flynn B, Foulds KE (2020) Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N Engl J Med 383(16): 1544-1355.
- DeFrancesco L (2020) COVID-19 antibodies on trial. Nat Biotechnol 38: 1242-1252.
- Zheng J (2020) SARS-CoV-2: An Emerging Coronavirus that Causes a Global Threat. Int J Biol Sci 16(10): 1678-1685.
- (2020) Whole genome of novel coronavirus, 2019-nCoV, sequenced.
- (2020) The Vaccine Quest. Scientific American. 322(6): 40-43.
- Korber B, Fischer WM, Gnanakaran S (2020) Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell 182(4): 812-827.
- Plante JA, Liu Y, Liu J (2020) Spike mutation D614G alters SARS-CoV-2 fitness and neutralization susceptibility. Nature.
- McAuley AJ, Kuiper MJ, Durr PA (2020) Experimental and in silico evidence suggests vaccines are unlikely to be affected by D614G mutation in SARS-CoV-2 spike protein. npj Vaccines 5: 96.
- Naqvi AAT, Fatima K, Mohammad T (2020) Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: Structural genomics approach. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 1866(10): 165878.
- Meredith LW, Hamilton WL, Warne B (2020) Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. The Lancet Inf Dis 20(11): 1263-72.
- Jeyanathan M, Afkhami S, Smaill F (2020) Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 20(10): 615-632.
- Le TT, Cramer JP, Chen R (2020) Evolution of the COVID-19 vaccine development landscape. Nature Reviews Drug Discovery 19(10): 667-668.
- Chauhan G, Madou MJ, Kalra S (2020) Nanotechnology for COVID-19: Therapeutics and Vaccine Research. ACS Nano 14(7): 7760-7782.
- https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines.
- https://www.who.int/news/item/06-11-2020-who-icmra-joint-statement-on-the-need-for-improved-global-regulatory-alignment-on-covid-19-medicines-and-vaccines
- Bull S, Jamrozik E, Binik A (2020) SARS-CoV-2 challenge studies: ethics and risk minimisation. J Med Ethics. medethics-2020-106504.
- McNeil S (2020) Overview of Vaccine Efficacy and Vaccine Effectiveness. Canadian Center for Vaccinology.
- (2020) Peter Thielen of Johns Hopkins Applied Physics Laboratory in conversation with Hamilton Jon. How Mutations in The Coronavirus May Affect Development of a Vaccine.
- Jackson LA, Anderson EJ, Rouphael NG (2020) An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Med 383(20): 1920-1931.
- Ramasamy MN, Minassian AM, Ewer KJ (2020) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. The Lancet. 6736(20): 32466-32471.
- Tim Locke (2020) Are UK Doctors Sceptical of COVID-19 Vaccine? Medscape.com.
- Neumann Böhme S, Varghese NE, Sabat I (2020) Once we have it, will we use it? A European survey on willingness to be vaccinated against COVID-19. The European Journal of Health Economics 21(7): 977-982.
- Source: HCHE/ Uni Hamburg 2020 survey in Den, Fr, Netherland, PT, IT, UK, DNK. Link - https://www.hche.uni-hamburg.de/en/forschung/corona.html
- Yamey G, Schäferhoff M, Hatchett R (2020) Ensuring global access to COVID 19 vaccines. Lancet. 395(10234): 1405-1406.
- Halabi S, Heinrich A, Omer SB (2020) No-Fault Compensation for Vaccine Injury - The Other Side of Equitable Access to Covid-19 Vaccines. N Engl J Med. 383(23):e125.
- Dan JM, Mateus J, Kato Y (2020) Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. Preprint at bioRxiv.
- Wang JZ (2020) The potential for antibody-dependent enhancement of SARS-CoV-2 infection: Translational implications for vaccine development. J Clin Transl Sci pp. 1-4.
- Diamond MS, Pierson TC (2020) The challenges of vaccine development against a new virus during a pandemic. Cell Host and Microbe 27(5): 699-703.
- Lazarus JV, Ratzan SC, Palayew A (2020) A global survey of potential acceptance of a COVID-19 vaccine. Nat Med (2020). Pp.1-4.
- Reiter PL, Pennell ML, Katz ML (2020) Acceptability of a COVID-19 vaccine among adults in the United States: How many people would get vaccinated? Vaccine 38(42): 6500-6507.
- Bell S, Clarke R, Mounier Jacka S (2020) Parents’ and guardians’ views on the acceptability of a future COVID-19 vaccine: A multi-methods study in England. Vaccine 38(49): 7789-7798.

 Mini Review
Mini Review