Abstract
A high producer β-glucans strain of Saccharomyces cerevisiae was selected from our culture collection to evaluate its ability to assimilate selenium by growing it in YPD medium supplemented with inorganic sodium selenite. This strain was also used as a host to express the murine lactoferrin gene under the control of the promoter of the S. cerevisiae glyceraldehyde-3-phosphate dehydrogenase (GPD) gene. The yeast strain was cultivated to obtain biomass made up to of high β-glucans levels, the incorporated selenium and recombinant murine lactoferrin. This biomass was harvested and dried to obtain probiotic supplements T1 and T2. The amount of bioselenium and murine lactoferrin were determined in the resulting product and used to feed BALB/c mice for 30 days. Several parameters served to monitor evaluate the immune stimulatory effect and the physiological state of the animals during the test. Measurements were carried out at 0, 15th and 30th days. The results showed the composite supplement improves the physiological and immunological conditions of the tested animals compared to the control group. The results obtained pave the way for developing food supplements with similar characteristics for economically important species.
Introduction
Probiotics are live microorganisms that confer health benefits to the host when administrated in adequate amounts [1,2]. Yeasts are eukaryotic unicellular microorganisms belong to the fungus kingdom [3]. Saccharomyces cerevisiae metabolizes carbohydrates to carbon dioxide and alcohols in a process known as fermentation which is extensively known and used by humans in food and alcoholic beverages production since the beginning of human civilization [4]. S. cerevisiae has also been used as a model organism for biological research and biotechnological processes. In general, there are a great number of yeast species, widely distributed in the nature and its ecological role has been matter of extensive studies. Yeasts, particularly S. cerevisiae and S. boulardii, are used as a nutritional supplement for direct consumption in different forms. Yeast strains are used as probiotic fungi in order to improve health and physiological conditions in many living organisms including humans as well as other vertebrates [5]. They could act in many beneficial forms that include (i) the prevention of binding and adhesion to the intestinal epithelium of pathogens,
a. The inhibition of toxin binding to the components of the
membrane of epithelial cells,
b. Prevention of diarrhea,
c. Protection of the digestive tract from drug therapies,
d. Stimulation of the innate immune system and the antiinflammatory,
anti-stress and hepatoprotective processes,
among other benefits for the better physiological functioning of
the entire organism [6-8].
In addition yeasts are a rich source of numerous important nutriments as proteins, carbohydrates, vitamins and minerals. Many of these probiotic characteristics are due to the components of the yeast cell wall. The yeast cell wall has a complex structure composed by lipids, glycoproteins and covalently interconnected polysaccharides that comprise water- and alkali soluble fractions of alpha mannans, mannoproteins and β-glucans [9]. In general, β-glucans are one of the most important biopolymers in living organisms, widely presented in cell walls of fungi and plant cells [10-12]. Three types of β-glucans are present in the cell walls of higher plants: (i) β(1,3)-glucan, (ii) cellulose, and (iii) xyloglucan [11]. In yeast, three different β-glucans types are present and they have been classified according to their solubility and aggregation properties: alkali insoluble branched β(1-3)-glucans, acid soluble branched β(1-6)-glucans and alkali soluble branched β(1-3)- glucans.
Mannoproteins are glycoproteins with attached mannan
residues, constitute between 20 and 50% of the total proteins of
the cell wall, while the greatest contribution to this proportion lies
in β(1-6)-glucans (65-90 % of total β-glucans fraction) of the total
weight [13]. As previously mentioned, β-glucans has an important
healthy effect on vertebrates especially on mammalian organisms at
improving their innate and acquired immunity, and overall organic
response to environmental changes and adverse effects triggered
[14-17]. The molecular structure and composition of β-glucans
as well as their derived physicochemical characteristics are
determinant on physiological and immune functions of vertebrates.
In yeast and fungi, β glucans are mainly present β (1-3)-glucans
backbone bearing branches composed by β (1-6)-linked side
groups, which are very important to modulate in vertebrates both
immune responses, innate and adaptive [18,19]. Other features
determining their biological activity are molecular mass, solubility
and type of aggregation, their spatial folding and the resulting
tertiary structure as well as the relative charge they carry [8,18,20].
Selenium is an essential micronutrient and well antioxidant
naturally found in soil, water and in some foods. Selenium
compounds, although in trace quantities, are indispensable
for proper physiological functioning of vertebrate organisms.
The beneficial effects of selenium can be linked mainly with the
selenoproteins and their relevant role in the organisms such
as, endocrine, muscular, cardiovascular, nervous, reproductive,
antioxidant and immune functions [19, 20]. Clinical evidence
indicates that consumption of Se-supplemented diet can increase
phagocytosis and the activity of natural killer cells, in sheep and
humans respectively [21-26]. In vertebrates, the mentioned biological effects of this microelement are mostly attributed to the
insertion of selenium in a group of proteins, named selenoproteins
(SeP), where the selenocysteine as take as a truly 21st amino acid
residue. To the present, 25 genes coding for SeP have been identified.
In some cases, the SeP are enzymes with catalytic functions,
responsible for biological reactions of the reduction-oxidation type,
antioxidant defense, DNA repair systems, epigenetic processes,
and thyroid hormone metabolism [26]. Those are the cases of
three thioredoxin reductases (TRs), glutathione peroxidases (GPx),
methionine sulfoxide reductase (MsrB1), and 3 thyroid hormone
deiodases (DIs) [27].
Lactotransferrin also known as Lactoferrin (LF), is a 78 kDa iron-binging glycoprotein which belongs to the Transferrin Protein family Talalactoferrin and LTF [28]. These proteins are able to transport proteins which can bind two Fe3+ ions in association with the binding of an anion, usually bicarbonate. LF is commonly found in colostrum and milk of vertebrates but also, although in smaller quantities, in tears, nasal secretions, saliva and intestinal fluids [29-31]. LF has antibacterial and antiviral actions retarding their proliferation and even killing them due its iron-binding capacity that results in microbial membrane destabilization [30].This protein is also an important modulator of inflammatory processes and immune response, probably by cytokine and chemokine production as well as interaction with oxidative/antioxidative processes, regulating the production of intracellular levels Reactive Oxygen and Nitrogen Species (ROSN) that may cause damage to lipids, proteins and DNA. But oxidative stress has been linked to activation of immune system [30,32-34]. LF activates innate immune response through receptors located in on the surface of macrophages, inducing phagocytosis of exogenous subjects and, at the same time, stimulating adaptative immune response and promoting the activity of antigen-specific T cells. Colostrum is particularly important for newborn mammals during the first days of life, because their immune systems are not fully developed, and they are highly susceptible to external pathogens and potentially allergenic substances [35]. Colostrum contains modulating factors that stimulate and regulate the immune response, including LF. We present the evaluation of probiotic supplement containing a combination of yeast β-glucans, organic selenium and lactoferrin in BALB/c mice during 30 days. The animals were monitored by determining blood, hepatic and renal biochemical markers related with specific organic functions, oxidative status, and immune system. The results showed an increase in immune response and improvement of tested organic function compared to the mice in control group and the synergistic action of the active components of the probiotic food supplement.
Materials and Methods
Probiotic Preparation
The normal diet consists in NUTRICUBOS-LabChows (Agribrands Purina, Mexico) and according to the nutritional values recommended by laboratory mice [36]. Probiotic food supplement (T1) composed by basic food (Normal Diet) supplemented by probiotic mixture containing a combination of yeast extract, insoluble β(1-3)- and β(1-6). glucan (final concentration 30%: weight), recombinant murine lactoferrin (final concentration 2.8 %: w), produced in yeast, and Se-enriched yeast extract (Se final concentration 0.015 % Se: weight). Probiotic food supplement (T2) has the same composition of insoluble β (1-3) and β (1-6) glucan (final concentration 30%: weight) but 33% less recombinant murine lactoferrin (final concentration 1.8 % %: w) and Se-enriched yeast extract (Se final concentration 0.01 % Se: weight). Recombinant lactoferrin was used as a dry yeast extract from transformed S. cerevisiae strain that expresses modified murine lactoferrin gene under the control of the promoter of the glyceraldehyde-3- phosphate dehydrogenase (GPD) gene, the level of LF production were established by immune dot blotting using our own anti-LF polyclonal antibodies and Protein A-peroxidase conjugate (AbCam, Cambridge, UK). The integrity of recombinant LF in of S. cerevisiae dry extract was verified by SDS-PAGE- 12.5% Electrophoresis (data not shown).
Animal Care, Husbandry and Probiotic Testing
Probiotic test experiment was performed in accordance with the Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research [37] and the Institutional Committee of Ethics, Animal Care and Welfare. Six-week-old female BALB/c mice were housed in temperature controlled (22-25ºC) on a 12h light/dark cycle (12h/12h) with access to water and food “ad libitium”. A total of 45 mice were divided into 3 groups of 15 mice each, first control group was fed a normal control diet, and testing groups was feed with a diet enriched with experimental probiotic supplement (T1, T2). The animals were weighed daily until day 30th.
Blood Biochemistry
Blood samples were collected through the ophthalmic plexus using a glass Pasteur pipette at the day 0 (prior to day exposure) and at the day 15th, 30th when the experimental test ended. Samples were centrifuged at 1850 x g for plasma collection. Liver and renal functions were evaluated by measuring, serum albumin, urea, uric acid, creatinine, alanine transaminase, aspartate transaminase, alkaline phosphatase, serum glutathione peroxidase and serum total antioxidant capacity. Measurements were done using the following commercial kits: a) Serum Albumin (QuantiChrom™ BCG Albumin Assay Kit, BioAssay Systems, Hayward, CA, USA); b) Urea BUN (Mouse Blood Urea Nitrogen ELISA Kit, Creative Diagnostics, Shirley, NY, USA); c) Uric Acid (QuantiChrom™ Uric Acid Assay Kit, BioAssay Systems, Hayward, CA, USA); d) Creatinine (Mouse Creatinine CREA ELISA, Kamiya Biomedical Co., Seattle, WA, USA); e) Alanine Transaminase (EnzyChrom™ Alanine Transaminase Assay Kit, BioAssay Systems, Hayward, CA, USA); f) Aspartate Transaminase (EnzyChrom™ Aspartate Transaminase Assay Kit, BioAssay Systems, Hayward, CA, USA); g) Alkaline phosphatase (Mouse Alkaline Phosphatase (ALP) ELISA, Kamiya Biomedical Co., Seattle, WA, US); h) Serum GSH-Activity (Mouse Glutathione (GSH) Colorimetric Cuvette Detection Kit (Innovative Research, MI, USA) and i) Plasma Total Antioxidant Capacity (Total Antioxidant Capacity Assay Kit, ABCAM, Cambridge, MA, USA).
Immunological Status
The immunological status of animals was followed up 0, 15th and 30th days of the probiotic assay by using 5 animals. The levels of lymphocytes, neutrophils and monocytes, were determined as well as the production of some cytokines were determined. Leucocytes numbers were established by using hemocytometer [38]; remaining parameters were evaluated by commercial kits.
Isolation of blood monocytes
Blood monocytes were extracted from two mL of peripheral blood from the wing vein of five selected animals from each experimental groups using standard procedure [39]. The total number of mononuclear-containing cells was counted by a standard hemocytometer and cell viability was determined.
Isolation of Bone Marrow derived macrophages
The five animals selected for monocytes isolation, were used to collect bone marrow cells according the standard described methodology [40,41].
Phagocytosis Assay
Peripheral blood monocytes and bone marrow derived macrophages from animals exposed to normal and probiotic supplemented feed during 30 days were subjected to the phagocytosis assay analysis. The assay was carried out by using commercial kit (Phagocytosis Assay Kit (Zymosan Substrate), AbCam, Cambridge, UK) 0, 15th and 30th of probiotic test. The external no engulfed Zymosan particles are blocked previously and the engulfed Zymosan particles react with a specific substrate to produce a colorimetric signal that can be detected by absorbance at 405 nm. The phagocytic index was calculated according to the following formula: phagocytic index = (total number of engulfed cells/total number of counted macrophages) × (number of macrophages containing engulfed cells/total number of counted macrophages) × 100 [42].
Cytokine Determination
Blood samples were drawn at 0, 15th and 30th days of the experiment. The blood was allowed to clot and serum was separated by centrifugation and transferred to new tubes for determination of both proinflammatory (Interleukin-2(IL-2), Interleukin 12 (IL-12) and gamma interferon (IFN-γ)) and antiinflammatory (Interleukin 4 (IL-4), Interleukin 10 (IL-10)) cytokines by using commercial kits (AbCam, Cambridge, UK).
Data Analysis
Data were analyzed using one-way ANOVA followed by Dunnett’s or Fisher’s protected least significant difference multiple comparison testing in SPSS13.0 (SPSS, Chicago, IL, USA). When necessary, data were transformed for normalization and to reduce heterogeneity of variance p-values <0.05 were statistically considered significant.
Results/Observations
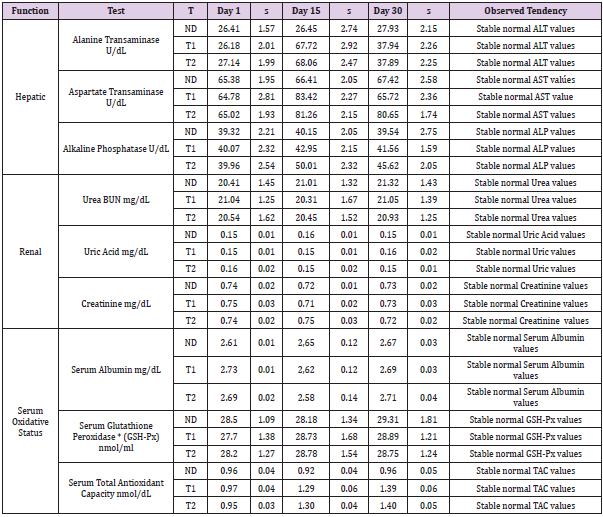
The Saccharomyces cerevisiae strain LX36 showed higher β-glucan content was selected from our culture collection to prepare probiotic food complement. The conditions for assimilation of selenium were optimized. This strain was transformed for constitutive expression of murine lactoferrin gene under the control of yeast Glyceraldehyde Phosphate Dehydrogenase (GPD) promoter. The same strain was used as a source of β-glucan, organic selenium and lactoferrin and became basic component of two probiotic supplements: T1 and T2. The probiotic mixture was completed with nutritional requirements recommended for mice US National Research Council (1995). During the probiotic three experimental group were fed with normal diet (ND), probiotic supplement 1 (T1) and probiotic supplement 2 (T2). The animals were monitored by determining blood, hepatic and renal biochemical markers related with specific organic functions, oxidative status and immune system. Table 1 shows the evolution of hepatic (Alanine Transaminase, Aspartate Transaminase, Alkaline Phosphatase), renal (Urea BUN, Uric Acid, Creatinine) and Serum Oxidative Status (Serum Albumin, Serum Glutathione Peroxidase, Serum Total Antioxidant Capacity) functions. We observe that mice in both, control and test groups are healthy and in a good physiological condition (Table 1).
Table 1: Blood biochemical tests performed in peripheral blood of BALB/c mice to evaluate hepatic, renal and serum oxidative status. ND: Control diet; T1: Supplemented Diet 1; T2: Supplemented Diet 2. n=5
The hepatic, renal and antioxidative functions did not suffer any significant changes and all values were normal. Immunological status of mice was monitored (Table 2). The number of lymphocytes, neutrophils, monocytes increases until the end of experiment in the animals fed with probiotic supplements T1 and T2 indicating a positive effect of both probiotic supplements on leucocytes proliferation. Significant stimulation effect was observed in phagocytosis assays of monocytes and macrophages. When animals were fed with probiotic supplement T1, phagocytosis in monocytes grew up from 35.68 % at day 0 to 57.21 % at 15th day and to 70.63% at the end of experiment. In case of mice fed with probiotic supplement T2 the same dynamic was observed, phagocytosis of monocytes shown an increment from 35.5 % at day 0, to 59.04 % at 15th day, and to 72.05% at the end of experiment. In the control group fed with normal diet the phagocytosis values remained without significant changes. The same tendency was observed in phagocytosis assay of macrophages fed with probiotic supplement T2. Phagocytosis grew up from 35.88 % to 67.34% and to 78.16 % at the end of the experiment when T1 given to animals, in case of T2 the phagocytosis index of macrophages increased from 36.02% at day 0 to 65.72 % at 15th day and 79.86% at day 30th.
Table 2: Analysis of immunological status, Leucocyte blood cells counts and cytokine production BALB/c mice. ND: Control diet;
The cytokine production during the experiment show stable values for Interferon gamma (IFN-γ), Interleukin 2 (IL2), Interleukin 12 (IL12) Interleukin 4 (IL4) and Interleukin 10 (IL10)in the control group. In case of the test groups increments were observed in all monitored cytokines but more significant in case of Interferon gamma (IFN-γ), Interleukin 12 (IL12) and Interleukin 10 (IL10). Values of IFN-γ at 30th day are 221% for T1 trail and 230% for T2 trail compared with the values found at day 0. The general health conditions of animals and weight increment were permanently observed and no differences were observed in both control and test group. Compared with the control group, at 30th day the increment in IFN-γ represented 214% (T1) and 209% (T2). A moderate increases are observed for the rest of monitored cytokines (Table 2). When compared the production of proinflammatory (IFNγ, IL2, IL12) with the production of antiinflammatory (IL 10, IL4) cytokines the increments observed at 15th and 30th days reflex a conserved balance between the production of both types of cytokines.
Discussion
Living organisms are in permanent interaction with internal
and environmental conditions and the ability to adapt the organic
processes to new reality is crucial for survival. Those processes
in animals frequently resulted in oxidative stress, metabolic
dysfunction, loss of essential nutriments, decreased availability
to maintain homeostasis of essential nutrients, energetic balance,
retain electrolytes, and overall physiological dysregulation leading
to poor health condition [43]. The beneficial effect of dairy diet
supplementation with probiotics is obvious when compare the
benefits evidenced in clinic and veterinary studies that comprise
the dynamic evolution of weight, liver, renal, immunological and
antioxidant markers. Probiotic supplements help support all
physiological functions since nutrition to the immune system
in healthy condition making any metabolic dysfunction and/or
infection less severe and helping the health recovery [26,44,45]. We
tested two probiotic supplements that include three yeast-derived
probiotic compounds: β-glucans, a component of yeast cell wall;
organic selenium, derived from selenized yeasts and recombinant
murine lactoferrin. We fed 45 BALB/c mice for 30 days divided in
three groups of 15 animals: control group fed with normal diet and
two test groups fed with probiotic mixtures T1 and T2.
The evolution and health status of animals was followed by
measuring marker vital functions, oxidative status and immune
system , including hepatic (Alanine Transaminase, Aspartate
Transaminase, Alkaline Phosphatase), renal (Urea BUN, Uric Acid,
Creatinine), Serum Oxidative Status (Serum Albumin, Serum
Glutathione Peroxidase, Serum Total Antioxidant Capacity), Immune
response (Quantification of Lymphocytes, Neutrophils, Monocytes,
Phagocytosis in Monocytes and Macrophages, Production of
proinflammatory (IFNγ, IL2, IL12) and antiinflammatory (IL4,
IL10) cytokines. Evaluations were made on days 0, 15th and 30th
days. The supplemented diets (T1 and T2) used for animal feeding
in this experiment contained identical nutritional value but differ in the proportion of β-glucans, organic selenium, and recombinant
murine lactoferrin. That proportion is 30% less in T2 compared with
T1. β-glucans are absent in vertebrate cells, and this is an important
fact in their overall stimulation effect on innate and acquired
immunity. The vertebrates lack β-glucans, they are recognized as
foreign invasive and potentially pathogenic substance as Microbe-
Associate Molecular Pattern (MAMPs) by Pattern Recognition
Receptors (PRR). These receptors, play a crucial role in the innate
immune system, and are mainly expressed by antigen presenting
cells such as dendritic cells, monocytes, macrophages, natural killer
cells, neutrophils, eosinophils and in epithelial cell of many tissues,
including intestinal epithelial cells [46,47].
In general, MAMPs include different agents such as bacterial
lipopolysaccharides or mannose; nucleic acids, such as bacterial or
viral DNA and RNA; peptidoglycan or lipoteichoic acid derived from
a Gram positive bacterium; formylmethionine and lipoproteins,
bacterial carbohydrates and β-glucans. The PRR are present mainly
in monocytes, macrophages, dendritic cells [47]. This fact can be
one of the reasons of the multifaceted action of β-glucans. Evidence
from in vitro and in vivo studies suggests that β-glucans have the
ability to promote the growth of beneficial microorganisms such as
Lactobacillus ssp. and Bifidobacterium ssp. in the gut microbiota
and the modulation of the immune system of mammals, including
humans [13,48,49]. Previously we studied the overall probiotic
effect of yeast β-glucans in mice after stress situation [8] and
hepatoprotective effects associated with their antioxidant capacity
[50]. This fact is relevant for keeping oxidant/antioxidant balance
in the respective organs, but also for maintaining the physiological
status of whole organism. In our experiment the hepatic, renal and
antioxidative functions didn’t suffer any significant changes from
normal standard values for mice. Albumin is as important factor
in an important homeostatic, nutritional and effective plasma pH
buffer [51]. Alterations in BSA levels may indicate liver, renal and
nutritional malfunctions and tested probiotic show no alteration of
mentioned functions.
The urea values in both control and test groups did not differ
and are normal for a healthy animal and the same results were
observed when uric acid was monitored; both are important
indicators of adequate kidney function probably because of
combinational effect of β-glucans, organic selenium and lactoferrin.
Urea, Uric acid Creatinine experiment indicated the good condition
of renal function in all animals of test and control groups. Levels
of Alanine Transaminase (ALT) and Aspartate Transaminase:
Aspartate Transaminase (AST), Alkaline Phosphatase (ALP) and
Glutathione peroxidase (GSH-Px) indicate a good liver function
[52,53]. These markers are related to a variety of pathophysiological
conditions, like inflammation and oxidative stress, renal and hepatic
malfunctions. The found normal levels evidenced that physiological
conditions didn’t affect animal health and have overall positive
effect [54,55]. Measurement of the total antioxidant capacity
(TAC) may be an important factor more to evaluate the ability of antioxidant response against oxidative stress damage [56]. Our
results showed normal levels in all animals as it was expected. TAC
assay is mostly focused on low molecular weight, chain breaking
excluding the contribution made by antioxidant enzymes and
metal-binding proteins [57].
These results suggest that the probiotic-supplemented diet had
a positive effect on antioxidative mechanisms, hepatic and renal
function and that the inclusion of combinational effects of three
active components (yeast β-glucans, organic selenium and lactoferrin)
in one probiotic supplement doesn´t affect hepatic, renal
and antioxidative functions in mice. The number of leukocytes
remains stable in the control during all the period of experiment
but increases were observed in test group fed with probiotic supplement.
The increases were not observed in the same proportion
among the different types of leukocytes. Lymphocytes grew up
from 12.08 to 16.31 and to 18.55 x 103cells/μl; neutrophils grew
up from 6.88 to 7.84 and to 8.86x 103cells/μl and monocytes grew
up from 0.79 to 0.89 and to 0.91 x 103cells/μl. These results suggest
that the probiotic-supplemented diet had a stimulatory effect
on the proliferation of different leucocyte populations providing
better protection against pathogens and infections and neoplastic
diseases [18,57,58]. Selenium was considered toxic element since
its discovery in 1857 until 1957 when its beneficial effects started
to be recognized and studied for the first time [26]. The beneficial
effects of Se are attributed at its in incorporation, instead of S, in the
cysteine residue of selenoproteins [59,60].
The way of action of selenium incorporated in form of
selenoproteins (SeP) over the different types of immune response
is not completely elucidated and is matter of many researches,
more exhaustive studies could be conducted but there are sufficient
evidences for its practical use in human and livestock healthcare.
In our experiments Se-supplemented diet increases phagocytosis
of monocytes and macrophage, improving antioxidative and
immunological responses [26,61-63]. As in the case of β-glucans, the
adequate selenium intake stimulates the innate immune systems.
This process includes the activation of macrophages through the
recognition of MAMPs (Microbe-Associate Molecular Pattern) by
PRR (Pattern Recognition Receptors), followed by activation of
blood leukocytes and modulation of inflammatory processes [64-
66] and enhancing proliferation and differentiation of CD4-T-helper
(Th) cells and improving T-cell receptor signals, and natural killer
(HK) cells [67,68]. In case of Lactotransferrin, in the intestinal
mucosa and neutrophils this protein is produced and secreted in
response to stimulation of inflammatory processes and in case of
infection the LF levels substantially grown in serum, stimulating
immune cells having LF receptors, like T- and B-cells, monocytes,
intestinal brush border membrane cells [3,69,70]. This protein
promotes the conversion of T-cells precursors to helper cells and
maturation of B-cells into Antigen Presenting Cells. The stimulation
of macrophages, dendritic cells and other immune response cells
keep stable the homeostasis in tissues where it’s present. These effects were fund also when combinational supplements T1 and
T2 were tested. It has been reported LF stimulates the production
of proinflammatory cytokines like TNF-α, Il-1β, IL-6 and IL-18 and
enhancing phagocytosis [30, 34,71].
We obtained that production of proinflammatory (IFNγ, IL2,
IL12) with the production of antiinflammatory (IL 10, IL4) cytokines
the increments observed at 15th and 30th days reflex a conserved
balance between the production of both types of cytokines as
a result combinational effect. LF also interacts with membrane
glycosaminoglycan, blocking the viral entry to host cells and/or
inferring subsequent viral transmission and probably enhancing
the activity of β-glucans [30,34,72,73]. It has been reported that
ingestion of probiotic supplements brings the described beneficial
effects on vertebrate organisms, but synergic combinational effect
of β-glucans has not been evaluated [17,25,32,61]. We obtained
the similar results with both supplements, the fact that same
positive and beneficial was reached using 30% less amount of
yeast β-glucans, organic selenium and lactoferrin indicate synergic
effect of all three components. The reported studies demonstrate
that they share some common mechanisms of action but at the
same time there are some particularities allowing overall synergic
activity over the different physiological functions of the vertebrates
[8,17,50,57,61].
Concluding Remarks
These results support the idea of systemic positive effect of dietary supplementation with probiotic in humans and animal. In animal production during handling operations that implies the manipulation of animals, change of feeding, transfers to another location, transport to a new region, etc., and changes in animal life cycle (birth, breastfeeding or breeding, weaning, calving, etc.). These drastic changes in living conditions and stages of the life cycle can have a dramatic impact on the productivity of the livestock by weight loss, slow weight gain, organ physiological malfunction and diminish of immunological defenses. We seek to minimize its consequences with dietary supplements as preventive treatment with h use of probiotic with several key compounds with the ability to overall activate multiple physiological mechanisms with overall beneficial effect to the organic vital functions and to the animal health.
References
- Suh SO, Mc Hugh JV, Pollock DD, Blackwell M (2005) The beetle gut: a hyperdiverse source of novel yeasts. Mycological Research109(3): 261-265.
- Sláviková E, Vadkertiová R(2003) The diversity of yeasts in the agricultural soil. Journal of Basic Microbiology 43(5): 430-436.
- Kurtzman CP, Piškur J (2005) Taxonomy and phylogenetic diversity among the yeasts. In: Sunnerhagen P., Piskur J. (Eds.). Comparative Genomics. Topics in Current Genetics 15, Springer, Berlin, Heidelberg, Germany.
- Legras J, Merdinoglu D, Cornuet J, Karst F (2007) Bread, beer and wine: Saccharomyces cerevisiae diversity reflects human history. Molecular Ecology 16(10): 2091-2102.
- Sen S, Mansell TJ (2020) Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genetics and Biology137: 103333.
- Kraswska A, Murzyn A, Dyjankiewicz A, Lukaszewicz M, Dyjankiewicz D (2009) Chemical composition of the cell wall of probiotic and brewer’s yeast in response to cultivation medium with glycerol as a carbon source. FEMS Yeast Res 497: 213-222.
- Vanhee LME, Goemé F, Nelis HJ, Coenye T (2010) Quality control of fifteen probiotic products containing Saccharomyces boulardii. Journal of Applied Microbiology 109: 1745-1752.
- De Jesus Torres Osorno A, López Mendoza FJ, De la Riva GA (2018) Isolation and Characterization of Wild Yeast Strains Producing Beta Glucans; Probiotic Systemic Effect in Mice under Conditions of Environmental Stress. Ann Food Process Preserv 1023: 1-14.
- Bzducha Wróbel A, Klieliszek M, Blazejak S (2013) Chemical composition of the cell wall o probiotic and brewer´s yeast n response to cultivation medium as a carbon source. Eur Food Res Technol 237: 489-499.
- Ruiz Herrera J, Ortiz Castellanos L (2019) Cell wall glucans of fungi. A review. The Cell Surface 5: 2468-2330.
- Hayashi T (1989) Measuring β-Glucan Deposition in Plant Cell Walls. In: Linskens HF, Jackson J F (Eds.). Plant Fibers. Molecular Methods of Plant Analysis, 10. Springer, Berlin, Heidelberg, Germany.
- El KhouryD, Cuda C, Luhovyy BL, Anderson GH (2012) Beta glucan: health benefits in obesity and metabolic syndrome. Journal of Nutrition and Metabolism pp. 851362.
- Songisepp E, Kals J, Kullisaar T, Mändar R, Hütt P, et al. (2005) Evaluation of the functional efficacy of an antioxidative probiotic in healthy volunteers. Nutrition Journal 4: 22.
- Doyon M, Labrecque JA (2008 Functional foods: a conceptual definition. British Food Journal 110(11): 1133-1149.
- Sen S, Mansell TJ (2020) Yeasts as probiotics: Mechanisms, outcomes, and future potential. Fungal Genet Biol.Fungal Genet Biol 137: 103333.
- Bohn JA, Be Miller JN (1995) (1-3) β-D-glucans as a biological response modifier: a review of structure-functional relationships. Carbohydr Polym 28(1): 3-14.
- Stier H, Ebbeskotte V, Gruenwald J (2014) Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutrition Journal 13: 38.
- Vetvicka V, Vetvickova J (2015) Glucan supplementation enhances the immune response against an influenza challenge in mice. Annals of Translational Medicine 3(2): 22.
- Rayman MP (2012) Selenium and human health. Lancet379 (9822): 1256-1268.
- Roman M, Jitaru P, Barbante C (2014) Selenium biochemistry and its role for human health. Metallomics 6(1): 25-54.
- Ravaglia G, Forti P, Maioli F, Bastagli L, Facchini A, et al. (2000) Effect of micronutrient status on natural killer cell immune function in healthy free-living subjects aged. Am J Clin Nutr 71(2): 590-598.
- Milad K, Racz O, Šipulová A, Bajová V, Kovac G (2001) Effect of vitamin E and selenium on blood glutathione peroxidase activity and some immunological parameters in sheep. Veterinarni Medicina 46: 1-5.
- Kassan S, Goenaga Infante H, Maharaj L, Hiley CT, Juliger S, et al. (2011) Methylselenicic acid inhibits HDAC activity in diffuse large B-cell lymphoma cell lines. Cancer Chem Pharmacol 68: 815-821.
- Bera S, De Rosa B, Rachidi W, Diamond AM (2013) Does a role for selenium in DNA damage repaor exlain apparent controversies in its use in chemoprevention? Mutagenesis 28: 127-134.
- Wang N, Tan HY, Li S, Xu Y, Guo W, et al. (2017) Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxid Med Cell Longev Epub 2017.
- Avery JC, Hoffmann PR (2018) Selenium, Selenoproteins, and Immunity. Nutrients 10(9): 1203.
- Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cellular and molecular life sciences: CMLS 66(15): 2457-2478.
- Kinkade JM, Miller W, Segars FM (1976) Isolation and characterization of murine lactoferrin. Biochim Biophys Acta 446(2): 407-418.
- Van der Strate BW, Beljaars L, Molema G, Harmsen MC, Meijer DK (2001) Antiviral activities of lactoferrin. Antiviral Research 52(3): 225-239.
- Legrand D, Pierce A, Elass E, Carpentier M, Mariller C, et al. (2008) Lactoferrin structure and functions. Advances in Experimental Medicine and Biology 606: 163-194.
- Siqueiros Cendón T, Arévalo Gallegos S, Iglesias Figueroa BF, García Montoya IA, Salazar Martínez J, et al. (2014) Immunomodulatory effects of lactoferrin. Acta Pharmacologica Sinica 35(5): 557-566.
- Baveye S, Elass E, Mazurier J, Spik G, Legrand D (1999) Lactoferrin: a multifunctional glycoprotein involved in the modulation of the inflammatory process. Clinical Chemistry and Laboratory Medicine 37(3): 281-286.
- De la Rosa G, Yang D, Tewary P, Varadhachary A, Oppenheim JJ (2008) Lactoferrin acts as an alarmin to promote the recruitment and activation of APCs and antigen-specific immune responses. Journal of Immunology (Baltimore, Md. 1950) 180(10): 6868-6876.
- Legrand D, Elass E, Carpentier M, Mazurier J (2005) Lactoferrin: a modulator of immune and inflammatory responses. Cellular and Molecular Life Sciences: CMLS 62(22): 2549-2559.
- Rovere Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, et al. (2004) HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO reports 5(8): 825-830.
- (1995) National Research Council (US) Subcommittee on Laboratory Animal Nutrition, Nutrient Requirements of Laboratory Animals: Fourth Revised Edition, 1995. National Academies Press (US). PMID: 25121259.
- (2012) Guidelines for Ethical Conduct in the Care and Use of Nonhuman Animals in Research. American Psychological Association (APA) (1912). Washington DC, USA.
- O Connell KE, Mikkola AM, Stepanek AM, Vernet A, Hall CD, et al. (2015) Practical murine hematopathology: a comparative review and implications for research. Comparative Medicine 65(2): 96-113.
- Houthuys E, Movahedi K, De Baetselier P, Van Ginderachter JA, Brouckaert P (2010) A method for the isolation and purification of mouse peripheral blood monocytes. Journal of immunological methods 359(1-2): 1-10.
- Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. Current Protocols in Immunology, Chapter 14, Unit-14.1.
- Weischenfeldt J, Porse B (2008) Bone Marrow-Derived Macrophages (BMM): Isolation and Applications. Cold Spring Harb Protoc 13(12).
- Jansen WT, Väkeväinen Anttila M, Käyhty H, Nahm M, Bakker N, et al. (2001) Comparison of a classical phagocytosis assay and a flow cytometry assay for assessment of the phagocytic capacity of sera from adults vaccinated with a pneumococcal conjugate vaccine. Clinical and Diagnostic Laboratory Immunology 8(2): 245-250.
- Villafuerte G, Miguel Puga A, Murillo E, Machado S, Manjarrez E, et al. (2015) Sleep Deprivation and Oxidative Stress in Animal Models: A Systematic Review. Oxidative Medicine and Cellular Longevity 1-15: 234952.
- Kanagasabapathy G, Malek SNA, Mahmood A, Chua K, Vikineswary S, et al. (2013) beta-rich extract from Plerotus sajor-caju (Fr) singer prevents obesity and oxidative stress in mice fed on high-fat diet. Evidence-based Complementary and Alternative Medicine: 1- 10.
- Vetvicka V, Vetvickova J (2018) Glucans and Cancer: Comparison of Commercially Available β-glucans - Part IV. Anticancer Research 38(3): 1327-1333.
- Weindl G, Wagener J, Schaller M (2010) Epithelial Cells and Innate Antifungal Defense. Journal of Dental Research 89(7): 666-675.
- Thomas M, Pierson M, Uprety T, Zhu L, Ran Z, et al. (2018) Comparison of Porcine Airway and Intestinal Epithelial Cell Lines for the Susceptibility and Expression of Pattern Recognition Receptors upon Influenza Virus Infection. Viruses 10(6): 312.
- Bigliardi B, Galati F (2013) Innovation trends in the food industry: The case of functional foods. Trends in Food Science & Technology 31(2): 118-129.
- Kamiya T, Tang C, Kadoki M, Oshima K, Hattori M, et al. (2018) β-Glucans in food modify colonic microflora by inducing antimicrobial protein, calprotectin, in a Dectin-1-induced-IL-17F-dependent manner. Mucosal Immunol 11: 763-773.
- De la Riva GA, López Mendoza F, Agüero-Chapin G (2018) Known Hepatoprotectors Act as Antioxidants and Immune Stimulators in Stressed Mice. Perspectives in Animal Health Care Curr Pharm Des 24(40): 4825-4837.
- Maxwell SRJ, Thomason H, Sandler D, Leguen C, Baxter MA, et al. (1997) Antioxidant status in patients with uncomplicated insulin-dependent and non-insulin-dependent diabetes mellitus. European Journal of Clinical Investigation 27(6): 484-490.
- Jin Y, Wang J, Pan X, Wang L, Fu Z (2013) cis-Bifenthrin enantioselectively induces hepatic oxidative stress in mice. Pesticide Biochemistry and Physiology 107(1): 61-67.
- Cichoż Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World Journal of Gastroenterology 20(25): 8082-8091.
- Battelli MG, Bolognesi A, Polito L (2014) Pathophysiology of circulating xanthine oxidoreductase: new emerging roles for a multi-tasking enzyme. Biochimica et Biophysica Acta 1842(9): 1502-1517.
- Kanbay M, Jensen T, Solak Y, Le M, Roncal Jimenez C, et al. (2016) Uric acid in metabolic syndrome: From an innocent bystander to a central player. European Journal of Internal Medicine 29: 3-8.
- Young IS (2001) Measurement of total antioxidant capacity. Journal of Clinical Pathology 54(5): 339.
- Matarese G, La Cava A (2004) The intricate interface between immune system and metabolism. Trends in Immunology 25(4): 193-200.
- Schaible UE, Kaufmann SH (2007) Malnutrition and infection: complex mechanisms and global impacts. PLoS Medicine 4(5): e115.
- Seyedali A, Berry MJ (2014) Nonsense-mediated decay factors are involved in the regulation of selenoprotein mRNA levels during selenium deficiency. RNA (New York NY) 20(8): 1248-1256.
- Lin HC, Yeh CW, Chen YF, Lee TT, Hsieh PY, et al. (2018) C-Terminal End-Directed Protein Elimination by CRL2 Ubiquitin Ligases. Molecular Cell 70(4): 602-613e3.
- Vetvicka V, Pinatto Botelho MF, Dos Santos AA, De Oliveira CA (2014) Evaluation of a special combination of glucan with organic selenium derivative in different murine tumor models. Anticancer Research 34(12): 6939-6944.
- Spengler G, Gajdács M, Marć MA, Domínguez Álvarez E, Sanmartín C (2019) Organoselenium Compounds as Novel Adjuvants of Chemotherapy Drugs-A Promising Approach to Fight Cancer Drug Resistance. Molecules 24(2): 336.
- Huang Z, Rose AH, Hoffmann PR (2012) The role of selenium in inflammation and immunity: from molecular mechanisms to therapeutic opportunities. Antioxidants & Redox Signaling 16(7): 705-743.
- Nelson SM, Lei X, Prabhu KS (2011) Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. The Journal of Nutrition 141(9): 1754-1761.
- Nelson SM, Shay AE, James JL, Carlson BA, Urban JF Jr, et al. (2016) Selenoprotein Expression in Macrophages Is Critical for Optimal Clearance of Parasitic Helminth Nippostrongylus brasiliensis. The Journal of Biological Chemistry 291(6): 2787-2798.
- Méplan C, Johnson IT, Polley AC, Cockell S, Bradburn DM, et al. (2016) Transcriptomics and proteomics show that selenium affects inflammation, cytoskeleton, and cancer pathways in human rectal biopsies. FASEB Journal: official publication of the Federation of American Societies for Experimental Biology 30(8): 2812-2825.
- Hoffmann FW, Hashimoto AC, Shafer LA, Dow S, Berry MJ, Hoffmann PR (2010) Dietary selenium modulates activation and differentiation of CD4+ T cells in mice through a mechanism involving cellular free thiols. The Journal of Nutrition 140(6): 1155-1161.
- Mahdavi M, Mavandadnejad F, Yazdi MH, Faghfuri E, Hashemi H, et al. (2017) Oral administration of synthetic selenium nanoparticles induced robust Th1 cytokine pattern after HBs antigen vaccination in mouse model. Journal of Infection and Public Health 10(1): 102-109.
- Donovan SM (2016) The Role of Lactoferrin in Gastrointestinal and Immune Development and Function: A Preclinical Perspective. The Journal of Pediatrics 173 (Suppl): 16-28.
- Lönnerdal B (2013) Bioactive proteins in breast milk. Journal of Pediatrics and Child Health 49( Suppl 1): 1-7.
- Latorre D, Puddu P, Valenti P, Gessani, S (2010) Reciprocal interactions between lactoferrin and bacterial endotoxins and their role in the regulation of the immune response. Toxins 2(1): 54-68.
- García Montoya IA, Cendón TS, Arévalo Gallegos S, Rascón Cruz Q (2012) Lactoferrin a multiple bioactive protein: an overview. Biochimica et Biophysica Acta 1820(3): 226-236.
- O Shea KM, Hwang SA, Actor JK (2015) Immune Activity of BCG Infected Mouse Macrophages Treated with a Novel Recombinant Mouse Lactoferrin. Annals of Clinical and Laboratory Science 45(5): 487-494.

 Research Article
Research Article