Abstract
The objective of this study is to examine the biological effects of Poynting Vector based torsion fields on mammalian cells in-vitro. M-1 mouse kidney collecting duct cells were placed into the torsion fields, with the biochemical and genetic indicators measured at 24 and 48 hours and then compared with those of the control group. The results indicate that the total adenosine pool (TAP), glutathione (GSH) levels, the genes of Hsf1 and Hsp32 were significantly increased, while the oxygen-containing free radicals and mitochondrial copies were significantly decreased. Many other factors remained unchanged including mitochondrial membrane potential (MMP) levels, superoxide dismutase (SOD) levels, telomerase, telomere length, cellular growth, cellular viability, and the genes of Bcl-2, P16, P21 and Sirt1. Based on the results, we conclude that mitochondria, which act as a cell signal transmission platform and can engage in intracellular signal processing, can be influenced by torsion fields. In addition, cell metabolic capacity, defense and stress resistance are significantly enhanced after short-term exposure to torsion fields.
Keywords:Mitochondria; Torsion fields; M-1 cells
Abbreviations: TAP: Total Adenosine Pool; GSH: Glutathione; MMP: Mitochondrial Membrane Potential; SOD: Superoxide Dismutase; EM: Electromagnetic; ROS: Reactive Oxygen Species; FBS: Fetal Bovine Serum; RPV: Rotating Poynting Vector; SD: Standard Deviation; OD: Optical Density
Introduction
The bioelectromagnetics community has repeatedly
demonstrated that Electromagnetic (EM) force fields affect
biological systems by modulating a variety of biochemical pathways
[1]. The Society for Bioacoustics has also clearly demonstrated
that acoustic sound energy has biological effects at the clinical and
cellular levels with a focus on ultrasound [2]. In addition to sound,
light at various wavelengths has a variety of biological effects [3]. In
addition to these classical forms of energy, there are non-classical
energy fields which do not obey the classical equations of motion
described by Maxwell [4]. One example is the longitudinal wave
(also called potential fields), which are modified EM fields [5].
Longitudinal waves exist in the human body [6] and exogenously
generated longitudinal waves have biological effects [7]. Another
type of non-classical, non-EM field is called a spinor field in the
western publications and the torsion field in the Russian literature.
Technically, a torsion field is a type of EM field with circular
polarization under torsion stress. These fields are described in the
physics literature in terms of their spin angular momentum [8] and
their polarization effects on the vacuum [9].
The electric charge and gravitational fields of all rotating objects
generates a torsion field, which have some unexpected properties
[10]. Similar to the gravitational field, the torsion field can’t be
shielded by any natural material. Unlike EM fields which propagate
along a transverse or longitudinal axis, torsion fields propagate
with clockwise (right handed) or counterclockwise (left handed)
spin. This is similar to other non-classical fields which propagate in
a helicoid [11] or spiral manner [12, 13]. Torsion fields propagate
through all medium but unlike EM fields, their amplitude does not fall off as 1/r2 [14], thereby allowing for non-local effects [10].
Theory further predicts that torsion fields do not possess energy or
mass, but only information. In this sense torsion fields are similar
to Qi as described below and longitudinal waves [15], as these
energies can be considered information field. Mitochondria are vital
organelles for an individual organism, mainly because of their role in
energy metabolism and biosynthesis. However, a growing number
of studies have shown that mitochondria, performing various
signal transduction functions, act as a platform to initiate cellular
signaling. Furthermore, mitochondria act as sensors and effectors
in multiple biological processes, including ATP and reactive oxygen
species (ROS) generation, related to cellular signaling pathways
[16]. In these processes, the mitochondrial outer membrane serves
as the primary signaling system which regulates cellular signaling
through mitochondrial dynamics and metabolites.
Most importantly, defects in mitochondria that regulate homeostasis and control cell signaling may be the basis of agerelated pathology [17] and many other diseases. As a cell signaling platform, mitochondria are constantly engaged in intracellular signal processing and transmission. This raises the question how mitochondria react and respond to signals outside the cell? Here we consider the possibility that such signals could be transferred via endogenous non-classical energy fields. To test this hypothesis, we investigated the effect of torsion fields on the energy metabolism of mouse kidney collecting duct cells (M-1). Reactive oxygen species, senescence related indexes such as telomerase, telomere length, TERT, Bcl-2, P16, P21 and other senescence related genes were also examined, to explore the possible influence of torsion fields on cell senescence.
Materials and Methods
Cell Culture
The M-1 mouse kidney collecting duct cell line was obtained from National Infrastructure of Cell Line Resource (Beijing, China, http://www.cellresource.cn) as a visceral cell which grows rapidly. The M-1 cells were seeded at a density of 5~6×105 cells/plate into 10cm plates and cultured as monolayers using DMEM/F12 1:1 medium supplemented with 10% fetal bovine serum (FBS) in a humidified 5% CO2 incubator at 37°C. The medium was changed 2–3 times per week. Stock cells were grown in 10cm culture dishes and split at a sub-cultivation ratio of 1:5.
Torsion Field Generator
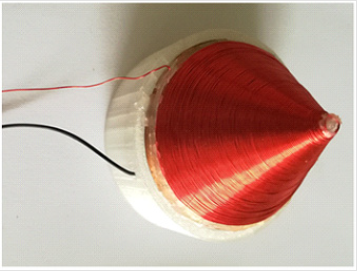
Torsion fields were generated using a RPV type emitter. The basic principle of Rotating Poynting Vector (RPV) generator, originally designed by Akimov [9], is based on the quantum spinspin interaction of orthogonal magnetic H and electric E fields generating a spiraling S-vector (Figure 1). We built our own RPV generator according to Akimov’s specifications, except a stronger neodymium magnet was placed at the bottom of the generator. The generator consists of a copper cone, with an underside diameter of 76mm and a height of 48.8mm (in according with the golden ratio). Between the copper cone and the copper coil (made of 23gauge solid copper wire) is a 2mm thick plastic cone, made by a 3D printer. When an electric signal is applied to the copper cone and the copper coil (see the two input wires in (Figure 1), a 106V/m electric field is generated, and whose direction is vertical to the inclined plane of the copper cone. The electric signal was generated by a high voltage AC positive pulse generator with a voltage of 2000-3000 Volts and a frequency of 13kHz. The electric current is not relevant here because in this generator only the voltage signal is used to generate the electric field between the copper cone and the copper coil. In this case, the component of the electric field will be orthogonal to the vertical magnetic field, a prerequisite to generate a torsion field. Therefore, a right-handed spiral RPV field will be generated around the plastic cone.
Application of Torsion Field to Cells
The M-1 cells were seeded at a density of 5~6×105 cells/plate into 10cm plates. After cultivating in the same incubator for 24h, triplicate plates with cells in the logarithmic growth phase were placed in another incubator containing the right-hand torsion field. Plates were stacked on top of each other on a shelf above the RPV generator, with the bottom plate about 4cm away from the tip of the RPV generator. For the control group, the triplicate plates were left in the original incubator for normal cultivation. Other than the torsion field treatment, these two incubators were set at exactly the same conditions, while being located in two separate rooms about 10 meters away. Cells for both the control and the treatment groups were incubated for 48h, with subsamples collected at 24h and 48h respectively during incubation.
Biochemical Analysis
Measurement of ATP, ADP, and AMP Levels: A new HPLC analysis was employed to study ATP, ADP, and AMP in M-1cells [18]. An aliquot of 1ml cell samples was centrifuged at 10,000 g for 5 min to collect cells, followed by the addition of 100μl PBS and 40μl deionized water. After that, 360μl perchloric acid (6%) was added to remove the protein by keeping samples on ice for an additional 10min. The cell extract was centrifuged at 10,000 g at 4°C for 5 min, with 300μl supernatant neutralized with 40μl of 2 M K2CO3, and filtered through a 0.45μm filter. A 10μl neutralized cell extract was used for determination of ATP, ADP, and AMP, which was carried outusing HPLC with mobile phase (0.1 M KH2PO4 buffer at pH 6.25) and 5% methanol (v/v) set at a rate of 0.6 ml/min and a detection wavelength of 254 nm. ATP, ADP and AMP quantization was calculated by computing peak areas, and injecting standard solutions of nucleotides with known concentrations. The total adenylate pool (TAP) was calculated by the following formula: [TAP]=[ATP]+[ADP]+[AMP]. Finally, TAP levels in cells were normalized to the total cell protein.
Measurement of ROS Levels: ROS levels were quantified in accordance with the protocol of the reactive oxygen species assay kit (Product No.: S0033; Beyotime, China). An aliquot of 0.5ml cell samples was incubated with DCFH-DA solution (2μM) at 37°C for 40min in the dark. Then samples were washed two times with PBS for measurement. The fluorescence intensity was recorded at 485nm (excitation) and 535nm (emission) using a micro plate reader (Tecan, Switzerland). Total ROS levels in cells were normalized to the total cell protein.
Measurement of Mitochondrial Membrane Potential: MMP levels were quantified in accordance with the protocol of the Mitochondrial Membrane Potential Assay Kit (Product No.: C2006; Beyotime, China). To study the MMP levels in cells, 0.5ml aliquots of the cell samples were incubated with a JC-1 probe at 37°C for 20 min in the dark. After the incubation, cells were washed twice with ice-cold JC-1 buffer solution. The JC-1 probe entering the mitochondrial matrix will aggregate and emit at 590nm (red) upon excitation at 525nm, whereas the monomeric JC-1 remaining in the cytoplasm emits at 530nm (green) upon excitation at 490nm. The fluorescence intensity was recorded using a microplate reader (Tecan, Switzerland). The value of MMP calculated as the ratio of red to green.
Measurement of Protein: Cell quantities were evaluated as the protein concentration using a Protein Assay Kit (Product No.:P0006C; Beyotime, China) with bovine serum albumin as the standard (0~1.5mg/ml). An aliquot of 0.5ml cell samples was centrifuged at 3000g for 5min to collect cells, which was then disrupted with Triton (0.1%) for 30min. After cell disruption, samples were centrifuged at 11000g for 5min to remove cell residues. The supernatant was used for optical density measurements at a wavelength of 595nm by using a microplate reader (Tecan, Switzerland).
Measurement of Cell Viability
Cell viability was evaluated by using the Cell Counting Kit-8 (CCK-8) (Product No.: C0038x; Beyotime, China). Briefly, an aliquot of 0.5mL cell samples was centrifuged at 3000g for 5min to collect cells, which were then re-suspended in 100μl culture medium and seeded in 96-well plates. Finally, 10μl CCK-8 was added to the wells and the plates were incubated for an additional 3h. The optical density (OD) was measured at a wavelength of 450nm using a microplate reader (Tecan, Switzerland).
Measurement of Antioxidant Levels: Antioxidant levels in cells were evaluated by the enzyme activity of SOD and the levels of GSH, using an SOD Assay Kit with WST-8 (Product No.: S0101; Beyotime, China) and a total Glutathione Assay Kit (Product No.: S0052; Beyotime, China) following the manufacturer’s protocols. The absorbance of SOD and GSH was assessed at 450nm and 412nm respectively using a microplate reader and the final SOD activity and GSH levels in cells were normalized to the total cell protein.
Mitochondrial DNA Quantification and Gene Expression Levels by q-PCR
Mitochondrial DNA Quantification: Total DNA was extracted from M-1 cells using the MiniBEST Universal Genomic DNA Extraction Kit (TaKaRa, 9765) according to the manufacturer’s protocols. The number of mtDNA copies was quantified by realtime PCR using TB Green® Premix Ex Taq™ II (TaKaRa, RR820) and a quantitative real time-PCR system (ABI, QuantStudio3). Primers specific for mitochondrion DNA and nuclear gene B2M are listed in (Table 1). The relative abundance of mtDNA copies was obtained by normalization to B2M using the 2-△△Ct method.
Gene Expression Levels: Total RNA was extracted from M-1 cells using the Mini BEST Universal RNA Extraction Kit (TaKaRa, 9767) according to the manufacturers’ protocols. RNA was then reverse transcribed into cDNA using Reverse Transcriptase M-MLV (TaKaRa, 2641A). Gene expression was quantified by real-time PCR using TB Green® Premix Ex Taq™ II (TaKaRa, RR820) and a quantitative real time-PCR system (ABI, Quant Studio 3). The primers specific for Hsf1, Hsp32, Bcl-2, P16, P21, Sirt1 and β-actin are listed in (Table 1). The relative abundance of each target was obtained by normalization to β-actin using the2-△△Ct
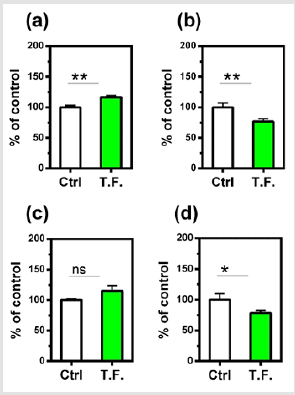
Figure 2: Effect of torsion field treatment on mitochondrial functions in M-1 cells
⦁ TAP (total adenylate pool, TAP=ATP+ADP+AMP,
ATP: adenosine triphosphate; ADP: adenosine
diphosphate; AMP: adenosine monophosphate);
⦁ ROS;
⦁ MMP;
⦁ Mitochondrial copies.
* P < 0.05; ** P < 0.01, when compared with the untreated
control using Student’s t-test.
Statistical Analysis Values of different measurements were
normalized to a respective mean control value from the untreated
samples and expressed as percent control. To evaluate treatment
effects, the results of treatments at 24h and 48h were combined
as a whole (divided into treated and control groups) for analysis.
All data were expressed as mean ± standard deviation (SD).
Comparisons between the control and treated groups were done
with the Student’s t-test using the GraphPad InStat software for
analysis, where P<0.05 was considered statistically significant.
Compared with the control group, there were many changes in
the cells that were treated by the torsion field, with a significant
increase in TAP production (Figure 2a), a significant reduction in ROS
levels (Figure 2b) and mitochondrial copies (Figure 2d). However,
the MMP remained unchanged (Figure 2c). This result indicated
that the torsion field significantly increased the metabolic capacity
while reducing the harmful oxygen-containing free radicals. The
observed decrease in mitochondrial copies, however, may be due
to environmental influences on the replication of mitochondrial
DNA. The influence of torsion field on the cellular self-protection
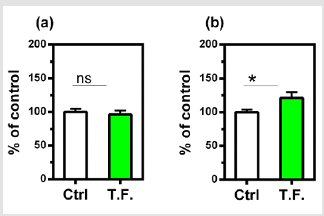
mechanisms was also investigated. As shown in (Figure 3), the SOD
showed no significant changes (a), but the GSH levels increased
significantly in treated cells (b), indicating an enhanced antioxidant
capacity of the cells.
Figure 3: Effect of torsion field treatment on antioxidant related items in M-1 cells
⦁ SOD;
⦁ GSH. *P < 0.05;
**P < 0.01, when compared with the untreated control
using Student’s t-test.
Figure 4: Effect of torsion field treatment on telomere related items in M-1 cells
⦁ telomerase;
⦁ telomere length.
*P < 0.05; **P < 0.01, when compared with the untreated
control using Student’s t-test.
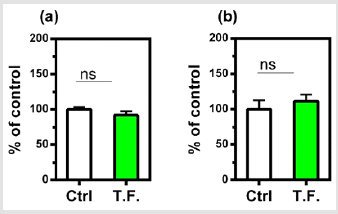
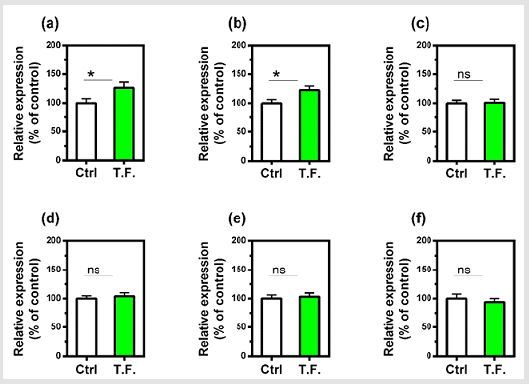
The effect of the torsion field on the telomeres of the cells was also investigated. Neither the telomerase nor telomere length in the cells showed a significant difference (Figure 4). It can be seen in (Figure 5), changes in cell growth and activity were not identified. After treatment with a torsion field, the gene expression in cells also varied, with a significant enhancement in Hsf1 and Hsp32 (Figure 5a & Figure 5b), but no changes for other genes (Bcl-2, P16, P21 and Sirt1) (Figure 5c-5f). The increase in these two genes indicates that the ability of cells to resist stress and maintain homeostasis is enhanced. This in turn promotes the self-protection of cells.
Figure 5: Effect of torsion field treatment on cellular growth and cellular viability in M-1 cells
⦁ cellular growth;
⦁ cellular visibility.
*P < 0.05; **P < 0.01, when compared with the untreated
control using Student’s t-test.
Figure 6: Effect of torsion field treatment on gene expression in M-1 cells
⦁ Hsf1;
⦁ Hsp32;
⦁ Bcl-2;
⦁ P16;
⦁ P21;
⦁ Sirt1.
Discussion
Although transverse EM fields are well established to effect biological systems. Circularly polarized light and torsion fields are examples of spinor fields. Spin waves, although studied in the physics literature, are not often used in biological experiments. Theory predicts that when torsion fields interact with matter, they only affect the spin state of matter. Russian scientists have a long history of research with torsion fields because they have a strong theoretical [9] and experimental [20] frame work and a way to generate torsion fields in the lab. In fact, there are several types of torsion fields and several types of generators to broadcast torsion fields. Of the different types of torsion field generators which have been scenically studied, generators based on the Poynting vector have been extensively studied [20, 21] and were used in the present study. A recent study by our group demonstrated some temperature anomalies with such torsion fields. However, the biological effects of these torsion fields have not been studied outside of Russia. Although there are numerous claims about the health benefits of right-handed torsion fields and the detrimental effects of lefthanded fields in the Russian literature. In general, left-handed fields accelerate life processes like germination [22] and right-handed fields reverse life process such as ripe peanut germination [23] and refolding of denatured proteins [24]. Unfortunately, there few studies have been published in peer-reviewed western journals
Even fewer studies are published regarding the biological effects of torsion fields. However, Lupichev demonstrated a non-local intercellular communication between cells in separate petri dishes [25]. Effects of torsion fields on the germination of Arabidopsis thaliana seeds, however, have been reported [22]. In this study, both right-handed and left-handed torsion fields stimulated seed germination by approximately 30%. A similar experiment with similar results was conducted by Szabo using Spruce seeds treated with an unconventional torsion field generator [26]. The present study is the first to demonstrate biological effects of Poynting Vector based torsion fields on mammalian cells in-vitro. The results indicate that ATP production and GSH levels were significantly increased, oxygen-containing free radicals were significantly decreased whereas SOD levels were unchanged in cultured mouse kidney cells treated with a home-made torsion field generator. This indicates that the antioxidant capacity of the cells was enhanced to mainly scavenge peroxide-based free radicals [27]. This conclusion is supported by the additional finding that torsion fields reduce ROS levels.
Nonetheless, these effects are target specific molecules, since telomerase and telomere length were not altered and are process specific, since cell growth was unaffected. Nonetheless, it is presently unclear whether torsion fields primarily interact with the plasma membrane or with intracellular proteins and organelles. However, the results of the present study indicate that torsion fields can interact directly with mitochondria. Mitochondria are well known to play an important role in the homeostasis regulation of cells. Thus, our results show that the mitochondrial mtDNA copy numbers are decreased significantly following the torsion field treatment, indicating the restricted replication of mitochondrial DNA. We also observed an increase in mitochondrial stability and cellular indicators related to longevity and aging (apoptosis) following torsion field treatment. However, torsion field’s effects on mitochondria are specific since the mitochondrial membrane potential (MMP) is unaffected. Nonetheless, mitochondria appear to be act as an antennae and are able to receive signals from torsion field which initiate the regulation of oxidative phosphorylation and superoxide free radical metabolism. At the nuclear level, our results indicate that torsion fields only affect specific genes. Thus, we observed a significant increase in Hsf1 and Hsp32 gene expression.
This indicates that at least in kidney cells, an enhanced ability of the cells to resist stress and maintain a steady state. The effects of torsion fields are specific since senescence genes (Bcl-2, P16, P21, Sirt1) showed no response. Although torsion fields are different than classical EM fields, as discussed in the introduction, their biological effects can be similar. Here, we demonstrated one type of torsion field stimulates TAP, and others have shown that EM fields, of a different frequency, can also increase ATP synthesis in erythrocytes [28]. In contrast to the anti-oxidant effects reported here, most EM fields have pro-oxidative capacity in-vitro [29]. We conclude that mitochondria, which act as a cell signal transmission plat form and can engage in intracellular signal processing, can be influenced by torsion fields. In addition, cell metabolic capacity, defense and stress resistance are significantly enhanced after short-term exposure to torsion fields. While mitochondrial DNA replication is limited, mitochondria stability is maintained and supported by indicators of longevity and aging (apoptosis), thus further confirming the huge role of mitochondrial homeostasis regulation.
References
- Hug K, Röösli M (2012) Therapeutic effects of whole‐body devices applying pulsed electromagnetic fields (PEMF): A systematic literature review. Bioelectromagnetics 33(2): 95-105.
- Baker KG, Robertson VJ, Duck FA (2001) A review of therapeutic ultrasound: Biophysical effects. Physical Therapy 81(7): 1351-1358.
- Holick MF (2016) Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Research 36(3): 1345-1356.
- Barrett TW (1993) Electromagnetic phenomena not explained by Maxwell's equations. In: Essays on the Formal Aspects of Electromagnetic Theory p. 6-86.
- Monstein C, Wesley JP (2002) Observation of scalar longitudinal electrodynamics waves. Europhysics Letters 59(4): 514-521.
- Yamato Y, Matsukawa M, Otani T, Yamazaki K, Nagano A (2006) Distribution of longitudinal wave properties in bovine cortical bone in vitro. Ultrasonics 44: e233-237.
- Rampl I, Boudný V, Cíž M, Lojek A, Hyršl P (2009) Pulse vector magnetic potential and its influence on live cells. 2009 International Conference on eHealth, Telemedicine, and Social Medicine Feb 1: 99-107.
- Hammond R (1994) Spin, torsion, forces. General Relativity and Gravitation 26(3): 247-263.
- Akimov AE, Tarasenko VY (1992) Models of polarized states of the physical vacuum and torsion fields. Russian Physics Journal 35(3): 214-222.
- Yurth DG (2000) Torsion field mechanics: Verification of non-local field effects in human biology. Journal of New Energy 5: 64-75.
- Lakhtakia A (1994) Time-dependent Beltrami fields in material continua. International Journal Infrared and Millimeter waves 15(2): 369-394.
- Fronsdal C (1958) On the theory of higher spin fields. Il Nuovo Cimento 9(2): 416-443.
- Tam AC, Happer W (1977) Attraction/repulsion of circularly polarized laser beams. Physics Review Letters 38(6): 278-284.
- Akimov AE (1997) Heuristic discussion of search for long range Interactions. The EGS-concepts. Journal of New Energy News 2(3-4): 59-80.
- Gao P (2016) Attempts to detect the torsion field nature of scalar wave generated by dual Tesla coil system. The fifth “Torsion Fields and Information Interaction” conference, Moscow.
- Esseltine JL, Scott JD (2013) AKAP signaling complexes: pointing towards the next generation of therapeutic targets? Trends in Pharmacological Sciences 34(12): 648-655.
- Mammucari C, Rizzuto R (2010) Signaling pathways in mitochondrial dysfunction and aging. Mechanisms of Ageing and Development 131(7-8): 536-543.
- Chen YJ, Wu XX, Li TJ (2018) A method for detecting the energy of organism.
- Malik AN, Czajka A, Cunningham P (2016) Accurate quantification of mouse mitochondrial DNA without co-amplification of nuclear mitochondrial insertion sequences. Mitochondrion 29: 59-64.
- Kernbach S (2013) Unconventional research in USSR and Russia: Short overview.
- Lunev VI (1995) Experimental research in the field of spin-torsion interactions (rus).
- Inoan SL, Criveanu H (2014) Influence of torsion fields on Arabidopsis thaliana seed germination. Agriculture-Science and Practice 89(1-2): 154-157.
- Li SC, Sun CL, Shen JC, Hou JZ (1999) Revival of peanut by psycho kinesis. Chinese Journal of Somatic Science 9(2): 52-54.
- Yuan TZ, Ormonde CF, Kudlacek ST, Kunche S, Smith JN, et al. (2015) Shear‐stress‐mediated refolding of proteins from aggregates and inclusion bodies. Chem Bio Chem 16(3): 393-396.
- Lupichev LN, Lupichev NL, Marchenko VG (1989) Distant interactions of material objects in Nature. Issledovaniye dinamicheskih svoistv raspredelyonnyhsred, Moscow p. 3-12.
- Szabo A, Criveanu HR, Miron CP, Micle S (2010) Unconventional germination methods of spruce (Picea abies) seeds using AD type bio-phyto–modulators. Bulletin of University of Agricultural Sciences and Veterinary Medicine Horticulture 67(2): 391-393.
- Ma XY, Deng D, Chen WD (2017) Inhibitors and activators of SOD, GSH-Px, and CAT. In: Enzyme Inhibitors and Activators Chapter 9: 207-224.
- Liu DS, Astumian RD, Tsong TY (1990) Activation of Na+ and K+ pumping modes of (Na, K)-ATPase by an oscillating electric field. Journal of Biological Chemistry 265(13): 7260-7267.
- Devrim E, Ergüder İB, Kılıçoğlu B, Yaykaşlı E, Çetin R, et al. (2008) Effects of electromagnetic radiation use on oxidant/antioxidant status and DNA turn-over enzyme activities in erythrocytes and heart, kidney, liver, and ovary tissues from rats. Toxicology Mechanisms and Methods 18(9): 679-683.

 Research Article
Research Article