Abstract
Background: Late dumping syndrome is a possible side-effect of gastric bypass. Hypoglycemic symptoms may develop 3-4 hours after certain types of foods. There may exist patients, however, who present hypoglycemia in the absence of dumping syndrome. The presence of only mild symptoms of hypo-glycemia may make the evaluation of these patients difficult and delay the identification of other possible sources of hyperinsulinemia, including an insulinoma.
Case Report: A 37-year-old woman underwent laparoscopic roux-en-Y gastric bypass (LRYGB) for morbid obesity. After operation, the patient had repeated episodes of hypoglycemia, diagnosed at follow-up as late dumping syndrome. The persistence of hypo-glycemic episodes after nutritional counseling and modifications in the feeding pattern led to consideration of an autonomous source of hyperinsulinemia, and MRI and endoscopic ultrasound identified insulinoma. After a laparoscopy and pancreatic tumor resection, she remains free of symptoms.
Conclusion: Hypoglycemic episodes after obesity surgery are not always related to dumping syndrome. The persistence of hypoglycemia despite nutritional counseling should raise the possibility that there may be other causes of dumping-like symptoms. Insulinoma, the most common cause of endogenous hyperinsulinemia, should be investigated in these patients, since it is a tumor that can be cured.
Keywords: Morbid Obesity, Bariatric Surgery, Gastric Bypass, Dumping Syndrome, Hypoglycemia, Insulinoma.
Introduction
Bariatric surgery is the most efficient treatment for morbid obesity. The combined properties of restrictive and malabsorptive gastric bypass lead to a low incidence of complications and to long-term weight loss [1]. Good results, however, depend on the bariatric surgeon and the multidisciplinary team dealing with the patients before and after surgery [2]. Physicians must be prepared to recognize complications and side-effects related to surgery. Dumping syndrome is a not uncommon complication of gastric bypass. The ingestion of calorie-dense, high-osmotic food followed by rapid emptying into the small intestine causes the release of peptides that induce tachycardia, palpitations, diaphoresis, and nausea [3]. Although these symptoms are considered side-effects of the surgery, their occurrence helps to limit the amount of food ingested, and, therefore, is one of the possible mechanisms for the good results of the gastric bypass. In most cases, the patients learn to avoid dumping syndrome by changes in quantity and quality of food ingested. We report the case of an obese patient who, after LRYGB, had started to experience symptoms of hypoglycemia. The patient was misdiagnosed as dumping syndrome and continued to have symptoms despite the nutritional counseling. Investigation found an insulinoma as the cause of hypoglycemia.
Case Report
37-year-old Caucasian woman was referred to our center
because of morbid obesity. She had tried to lose weight several
times, including with medications, but never succeeded. Her
incapacity in adhering to different nutritional approaches
associated with a persistent feeling of hunger became worse
in the past year. Her height was 179cm and body weight 130kg
Body Mass Index (BMI ) = 41 kg / m2 . She underwent an Oral
Glucose Tolerance Test (OGTT), which demonstrated deep
hypoglycemia after 90min, she received nutritional counseling returned 3 months later, with further weight gain of 7kg, unable
to follow the prescribed diet. She was then referred to our center
and she passed LRYGB. She lost weight (BMI 25), but 6 years later
she complained of episodes of nausea, tachycardia, and vomiting.
However, she did not associate these with feeding. She was
diagnosed as having late dumping syndrome and was advised to
decrease the ingestion of sweets and high osmolar fluids and to
increase the number of meals per day. The patient continued to have
these symptoms despite all changes in the eating pattern. She was
then referred to an endocrinologist for further investigation. Since
no response was observed after nutritional counseling, biochemical
tests were performed to investigate hyperinsulinemia.
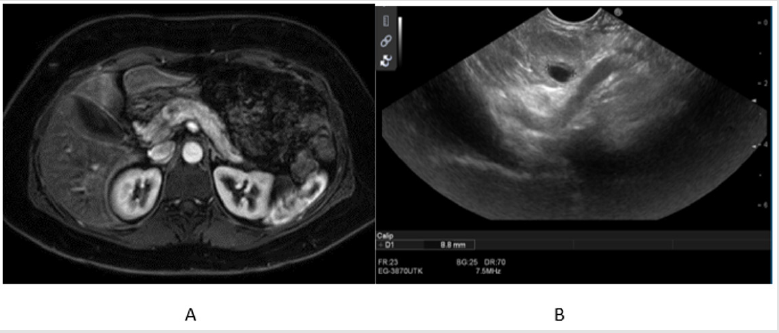
MRI and endoscopic ultrasound were performed, and both
demonstrated the presence of a Well differentiated endocrine
pancreatic neoplasia. (diameter 1.8cm) of approximately 1.8cm in
the body of the pancreas without lympho-vascular or perineural
invasion. (Figures 1A & 1B). A laparoscopy was performed, and an
insulinoma was identified and enucleated. After surgery, there have
been no further hypoglycemic episodes or symptoms.
Figure 1:
a. Magnetic resonance imaging and
b. Endoscopic ultrasound scans of the abdomen showing the presence of an insulinoma in the body of the pancreas.
Discussion
Insulinomas are the most common source of hyper-insulinemic
hypoglycemia [4]. The occurrence of hypoglycemic episodes,
although strongly suggestive of insulinoma, may be underestimated
in some patients, delaying the identification of the disease for up
to 5 years [5]. Even though neuroglycopenic symptoms are the
most known, some patients may present only mild symptoms or
signs, including weight gain in approximately 40% of the patients
[5]. Clinical and biochemical data from our patient suggest that
an insulinoma could have been one of the possible causes for the
weight gain and lack of response to clinical treatment of obesity
before bariatric surgery. The presence of hypoglycemia during
oral glucose tolerance test may indicate that, at that time, the
patient already had an autonomous source of insulin, which was
missed due to the major concern with weight gain and insulin
resistance. Moreover, persistent hyperinsulinemia in morbidly
obese patients is almost always suggestive of insulin resistance.
The elevated insulin levels in these patients are usual. Thus, only a
prolonged fast test would allow a better distinction between insulin
resistance and autonomous hyperinsulinemia. Lack of awareness of
symptoms of hypoglycemia by the patient may also result in delay
in the identification of the insulinoma. These patients often fail to
recognize autonomic warning symptoms. Therefore, hypoglycemia
is only detected through the presence of neuroglycopenic symptoms.
This situation has been described in patients with insulinomas
[6,7] as in a patient after vagotomy and gastric resection for ulcer
recurrence [8].
It has been suggested that this lack of awareness of
hypoglycemia may be due to a generalized central system
adaptation after repeated episodes of hypoglycemia [9,10]. If the
patient did have the insulinoma before surgery, she may have
been suffering episodes for a long time, which may have ultimately
resulted in her unawareness of episodes. Also, the persistent feeling
of hunger reported by the patient as the cause of her inability to
follow the prescribed diets may be an indirect sign of persistent
hypoglycemia. Hypoglycemic symptoms in obese patients after
bariatric surgery are usually related to dumping syndrome. Late
dumping syndrome is characterized by reactive hypoglycemia
secondary to hyperinsulinemia. Symptoms, mainly perspiration,
shakiness, difficulty in concentrating, decreased consciousness,
and hunger [11] occur 2-3 hours after feeding and are related
to glucagon-like peptide-1 (GLP-1), gastro-intestinal inhibitory peptide (GIP), and high glucagon levels [12,13]. These symptoms
are expected in the early phases of follow-up after gastric bypass.
Therefore, their occurrence is usually considered normal and no
further investigation is performed. The frequency and severity
of symptoms usually ameliorate with nutritional counseling and
modifications in the feeding pattern.
Some patients who present late dumping syndrome require
medications to control the hypo-glycemia. During the post-surgery
period, our patient received nutritional counseling as an attempt to
control the hypoglycemic episodes. She was advised to reduce the
amount of carbohydrate ingested and to increase the number of tiny
meals. It is interesting to speculate that these recommendations
may have worsened the episodes. Since the desirable effect was not
achieved, the patient was referred to an endocrinologist for further
evaluation. The prompt identification of fasting hypoglycemia
and hyperinsulinemia indicated that there may be an alternative
cause for hypoglycemia instead of late dumping syndrome.
The endoscopic ultrasound and MRI were then performed and
identified the pancreatic tumor as the organic cause of the
hypoglycemia. A laparoscopy was performed, and the insulinoma
was enucleated. It is important to consider that symptoms of
dumping are usually nonspecific, resembling those observed in
hypoglycemic patients. Based on this, nutritional counseling should
always be the first therapeutic intervention for patients with such
symptoms. However, if the symptoms persist, careful investigation
for an autonomous source of insulin should be performed before
beginning pharmacologic therapy for dumping syndrome. Patients
with persistent hypoglycemic episodes should have glucose, insulin
and C-peptide levels determined after a 12-hour fast, to exclude an
autonomous source of insulin secretion. In cases where these tests
are considered still doubtful, the patient should be hospitalized,
and the test should be repeated after a prolonged fast.
Conclusion
Bariatric surgeons should be aware of metabolic conditions including hypoglycemia, as a treatable cause of dumping-like symptoms.
References
- Dayan D, Kuriansky J, Abu Abeid S (2019) Weight Regain Following Roux-en-Y Gastric Bypass: Etiology and Surgical Treatment. Isr Med Assoc J 12(21): 823-828.
- Giusti V, Suter M, Héraief E, R C Gaillard, P Burckhardt (2001) Rising role of obe-sity surgery caused by increase of morbid obesity, failure of conventional treatments and unrealistic expectation: Trends from 1997 to 2001. Obes Surg 13(5): 693-698.
- Kellum JM, Kuemmerle JF, O’Dorisio TM, P Rayford, D Martin, et al. (1990) Gastrointestinal hormone response to meals before and after gastric bypass and vertical banded gastro-plasty. Ann Surg 211(6): 763-771.
- Matej A, Bujwid H, Wroński J (2016) Glycemic control in patients with insulinoma. Hormones (Athens) 15(4): 489-499.
- Dizon AM, Kowalyk S, Hoogwerf BJ (1999) Neuroglycopenic and other symptoms in patients with insuli-nomas. Am J Med 106: 307-310.
- Krysiak R, Okopień B, Herman ZS (2007) Wyspiak wydzielajacy insuline [Insulinoma]. Pol Merkur Lekarski 22(127): 70-74.
- Brown E, Watkin D, Evans J, Yip V, Cuthbertson DJ (2018) Multidisciplinary management of refractory insulinomas. Clin Endocrinol (Oxf) 88(5): 615-624.
- Tóth M, Szücs N, Jakab Z, Doros A, Nemes Z, et al. (2011) Malignus insulinoma [Malignant insulinoma]. Orv Hetil 152(10): 398-402.
- Bellini F, Sammicheli L, Ianni L, C Pupilli, M Serio, et al. (1998) Hypoglycemia unawareness in a patient with dumping syndrome: report of a case. J Endocrinol Invest 21: 463-467.
- Boyle PJ, Kempers SF, OConnor AM, R J Nagy (1995) Brain glu-cose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N Engl J Med 333(26): 1726-1731.
- Bertolotti A, Borgogna M, Facoetti A, Marsich E, Nano R (2009) The effects of alginate encapsulation on NIT-1 insulinoma cells: viability, growth and insulin secretion. In Vivo 23(6): 929-935.
- Mulla CM, Storino A, Yee EU, Lautz D, Sawnhey MS, et al. (2016) Insulinoma After Bariatric Surgery: Diagnostic Dilemma and Therapeutic Approaches. Obes Surg 26(4): 874-881.
- Tarchouli M, Ali AA, Ratbi MB, Belhamidi Ms, Essarghini M, et al. (2015) Long-standing insulinoma: two case reports and review of the literature. BMC Res Notes 8: 444.

 Case Report
Case Report