Abstract
Background: Extracorporeal Membrane Oxygenation (ECMO) is considered as the last rescue treatment for patients with acute respiratory distress syndrome (ARDS).
Methods: All nine patients with coronavirus disease 2019 (COVID-19) receiving ECMO therapy from two hospitals during the COVID epidemic in China were included in this study. The dynamic changes of clinical characteristics around ECMO initiation (±48 hours) were compared between survivors (n=4) and non-survivors (n=5).
Results: Of the nine patients, mean age was 64.7 y, 7 (77.8%) were men and 4 were survival to discharge. Blood group a seemed associated with elevated mortality in COVID-19 patients receiving ECMO support. The Pao2/Fio2 ratios higher than 150 mm Hg, decreased lactic acid and neutrophil-to-lymphocyte ratio (NLR), and increased lymphocyte count after 48-hour ECMO support and ECMO initiation at the rising period of C-reactive protein (CRP) were significantly associated with the outcome of survival in COVID-19 patients.
Conclusion: Non-A blood group, an increasing CRP level before ECMO initiation and Pao2/Fio2 rising to higher than 150 mm Hg and decreasing lactic acid and NLR levels after 48-hour ECMO support may be associated with survival outcome in COVID-19 patients receiving ECMO therapy. Further study with larger sample size is needed to validate these clinical experiences.
Keywords: Extracorporeal Membrane Oxygenation; Acute Respiratory Distress Syndrome; Coronavirus Disease 2019; ABO Blood Group; Pao2/Fio2 ratio; Lactic acid, Neutrophil-to-lymphocyte ratio, C-reactive protein
Abbreviations: A-aO2: Alveolar–Arterial Gradient; APACHE II: Acute Physiology and Chronic Health Evaluation II; ARDS: Acute Respiratory Distress Syndrome; CFR: Case Fatality Rate; COPD: Chronic Obstructive Pulmonary Disease; COVID-19: coronavirus Disease 2019; CRP: C-reactive protein; ECMO: Extracorporeal Membrane Oxygenation; Fio2: Fractional Of Inspired Oxygen; JZCH: Jingzhou Central Hospital, Hubei Province; NLR: Neutrophil-to-lymphocyte ratio; PaCO2: Partial Pressure Of Carbon Dioxide; Pao2: Partial Pressure Of Oxygen; PH: Power Of Hydrogen; PHSZ: The Third People’s Hospital of Shenzhen; SARS-CoV-2: Severe Acute Respiratory Syndrome Coronavirus 2; SOFA: Sequential Organ Failure Assessment
Introduction
The novel coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic at the beginning of 2020 and affected almost all of the human beings on this planet directly or indirectly. Our health systems, especially in the epicenters, such as Wuhan in China and New York City in the US, faced unprecedented challenges due to the enormous number of cases rising exponentially [1,2]. A report including approximately 72,314 cases from the Chinese Center for Disease Control and Prevention demonstrated that over 5% of COVID-19 patients developed severe pneumonia and had a high risk of acute respiratory distress syndrome (ARDS) [1,3]. Patients with ARDS having high mortality rate need Extracorporeal Membrane Oxygenation (ECMO) to support gas exchange, if their clinical condition cannot be improved by conventional mechanical ventilation. A recent study showed that only half of the COVID-19 patients receiving ECMO support were survival [4]. However, it remains unclear how to identify the patients who may benefit more before the ECMO therapy. In this study, we aimed to summary the factors related to the outcomes of COVID-19 patients receiving ECMO therapy, which may help the health workers in intensive care unit (ICU) make decisions on choosing the most appropriate treatment for a particular COVID-19 patient with ARDS.
Method
Study Design and Participants
This is a two-center, retrospective, observational study
conducted at Jingzhou Central Hospital, Hubei Province (JZCH)
and The Third People’s Hospital of Shenzhen (PHSZ), Guangdong
Province, China, which are the designated hospitals for COVID-19
patients. A total of nine COVID-19 patients received ECMO support
during the pandemic (three patients in JZCH and six patients in
PHSZ). All of these nine patients were included in this study. These
patients were managed by the experts from Guangdong Provincial
People’s Hospital (GDPH), which started ECMO therapy on 2006
and was certified by the Extracorporeal Life Support Organization
(ELSO). The medical record of each participant was retrieved from
the hospitals. Two well-trained physicians (HB and XL) reviewed the
medical records and extracted data independently and then crosschecked
the datasets. The data we collected include demographic characteristics, clinical symptoms, comorbidities, laboratory
findings, treatment during hospitalization, and discharge status.
This study was approved by the ethnic committees of GDPH,
JZCH, and PHSZ. Patients or families provided informed consent for
data analysis with anonymized individual data.
ECMO Therapy Procedure
In this study, COVID-19 was diagnosed based on chest scan and nucleic acid assay according to the World Health Organization Interim Guidelines [5]. ARDS was confirmed using the Berlin definition [3]. The patients received venovenous ECMO support when either partial pressure of oxygen (Pao2)/fractional of inspired oxygen (Fio2) <100 mm Hg or power of hydrogen (PH) <7.25 and partial pressure of carbon dioxide (PaCO2) >60 mm Hg over 6 hours. During ECMO therapy, the blood flow and oxygen flow were adjusted to maintain peripheral capillary oxygen saturation >90% and mixed venous oxygen saturation >70%. Pressure controlled ventilation strategy was adopted during ECMO therapy with settings of pressure < 25 cm H2O, positive end-expiratory pressure (PEEP) 10–15 cm H2O, respiratory rate 4–10 breaths per minute, and Fio2 less than 50%. All the patients accepted heparin continuous IV infusion to maintain activated clotting time (ACT) at 160–200 seconds and activated partial thromboplastin time (APTT) no more than two times of the upper limit of normal.
Statistical Analysis
Continuous variables were presented as mean with standard deviation (SD). Categorical variables were described as frequency with percentage. Fisher’s exact test for categorical variables and Wilcoxon-Mann-Whitney U test for continuous variables were used to compare the differences between survivors and non-survivors. Statistical analyses were performed using SPSS (version 25.0). P value of less than 0.05 was regarded statistically significant.
Results
Characteristics of the Patients with ECMO Therapy
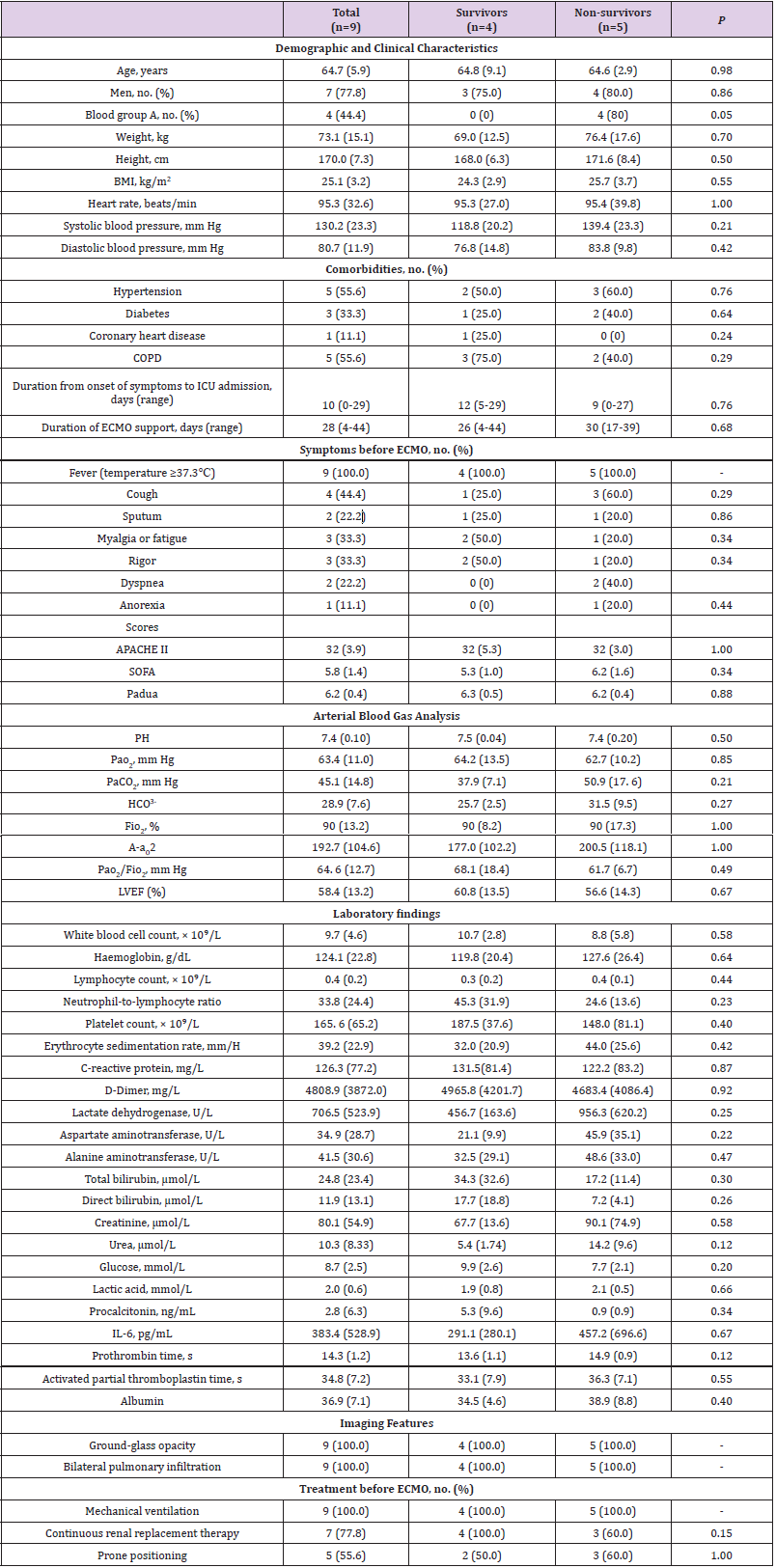
The general characteristics of the 9 COVID-19 patients who received ECMO therapy are described in (Table 1). Of the nine patients, four were survival to hospital discharge, mean age was 64.7 y, and seven (77.8%) were men. None of the four survivors had blood group A, however, 4/5 of non-survivors had blood group A (P=0.048). The most common comorbidities in these patients were hypertension (55.6%) and chronic obstructive pulmonary disease (COPD, 55.6%). Fever (100%) and cough (44%) were the most common symptoms on admission. Critical care scoring systems showed that the patients had mean Acute Physiology and Chronic Health Evaluation II (APACHE II) of 32, Sequential Organ Failure Assessment (SOFA) of 6, and Padua of 6 on the day of receiving ECMO support. The patients received ECMO support for 4-44 days. All of the patients showed ground-glass opacity in bilateral lung with pulmonary infiltration. Of the nine patients, all received mechanical ventilation, seven had continuous replacement therapy, and five used prone positioning before ECMO support.
Table 1: General characteristics of COVID-19 patients with acute respiratory distress syndrome before extracorporeal membrane oxygenation therapy.
Note: A-aO2, Alveolar–arterial gradient; APACHE II, Acute Physiology And Chronic Health Evaluation II; BMI, body mass index; COPD, Chronic obstructive pulmonary disease; Fio2, fraction of inspired oxygen; HCO3-, Bicarbonate; ICU, intensive care unit; IL-6, interleukin-6; LVEF, Left ventricular ejection fraction; PaCO2, Partial pressure of carbon dioxide; Pao2, Partial pressure of oxygen; SOFA, Sequential Organ Failure Assessment.
Laboratory Tests of the Patients on Day 0 of ECMO Therapy
Generally, the patients included in this study had elevated hemoglobin [124.1 (22.8) g/dL], erythrocyte sedimentation rate [39.2 (22.9) mm/H], CRP [126.3 (77.2) mg/L], D-dimer [4808.9 (3872.0) mg/L], lactic dehydrogenase (LDH) [706.5 (523.9) U/L], total bilirubin [24.8 (23.4) μmol/L], direct bilirubin [11.9 (13.1) μmol/L], glucose [8.7 (2.5) mmol/L], procalcitonin [2.8 (6.3) ng/ mL], and IL-6 [383.4 (528.9) pg/mL], and decreased lymphocyte count [0.4 (0.2) × 109/L] and urea [10.3 (8.33) μmol/L].
Dynamic Changes of Variables around ECMO Initiation (±48 hours)
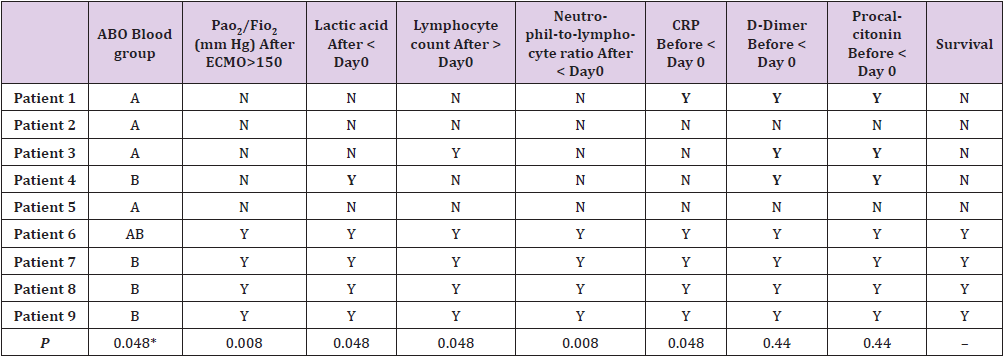
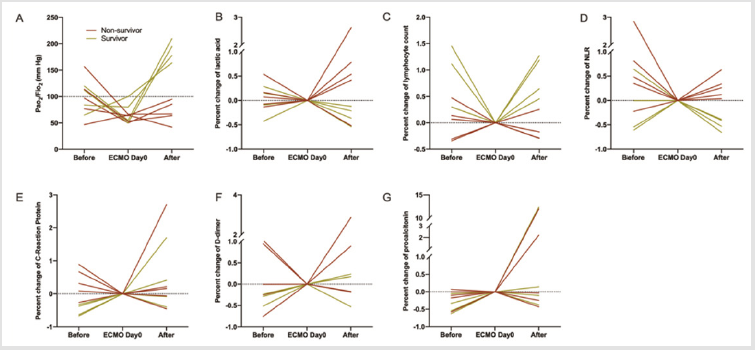
To identify the factors which may be associated with prognosis of COVID-19 patients treated with ECMO, we depicted the dynamic changes of Pao2/Fio2 ratio, lactic acid, lymphocyte count, neutrophilto- lymphocyte ratio (NLR), CRP, D-Dimer, and procalcitonin around ECMO treatment (Figure 1). Expect for Pao2/Fio2 ratio, the measurements 48-hour before ECMO and 48-hour after ECMO were normalized as percent changes of measurements at ECMO day 0. As shown in (Figure 1A), the nine patients had comparable Pao2/Fio2 ratio before ECMO therapy, however, the Pao2/Fio2 ratios of survivors recovered to higher than 150 mm Hg and the Pao2/Fio2 ratios of non-survivors were still lower than 100 mm Hg (P=0.008, (Table 2). The plasma lactic acid level of the four survivors decreased after 48-hour ECMO support, while most of the non-survivors (4/5) had increased lactic acid after ECMO therapy (Figure 1B); P=0.048, (Table 2). All of the four survivors had elevated lymphocyte count after 48-hour ECMO therapy, in contrast, 4/5 of the non-survivors had decreased lymphocyte (Figure 1C); P=0.048, (Table 2). The NLR of the four survivors decreased after 48-hour ECMO support, however, all of the five non-survivors had increased NLR after ECMO therapy (Figure 1D); P=0.008, (Table 2). In addition, the survivors received ECMO support when CRP was in rising period (Day 0 > Before ECMO), however, most of the non-survivors (4/5) received ECMO therapy when CRP was in declining period (Day 0 < Before ECMO) (Figure 1E); P=0.048, (Table 2).
Table 2: Association of ABO blood group and the change patterns of clinical characteristics with outcome of extracorporeal membrane oxygenation support in COVID-19 patients with acute respiratory distress syndrome.
Note: P values were calculated by Fisher’s Exact Test. *ABO blood group was converted to “A” or not before calculating P value
Figure 1: Changes of clinical characteristics in the individual patients 48 hours before, on the day of, and 48 hours after extracorporeal membrane oxygenation initiation.
As for D-dimer and procalcitonin, no obvious differences were found between the survivors and the non-survivors around ECMO support (Figures 1F & 1G). The absolute values of these measurements are shown in an additional file [see Additional file 1]. According to the results in (Table 2), the combination of ABO blood group, Pao2/Fio2, lactic acid, lymphocyte, and CRP may be related to the outcome of ECMO therapy.
Discussion
In this study, we described the characteristics of COVD-19 patients with ARDS receiving ECMO support from two hospitals and found that 44.4% (4/9) were survival to discharge. Furthermore, our results demonstrated that ABO blood group and patterns of changes of Pao2/Fio2, lactic acid, lymphocyte count, NLR, and CRP around ECMO initiation (±48h) may be related to the outcome of ECMO therapy in COVID-19 patients. ECMO is considered as the last rescue treatment for COVID-19 patients with ARDS, although the effect of ECMO therapy on the management of COVID-19 remains unclear at the current stage [6,7]. The case fatality rate (CFR) of COVID-19 has been reported to be 4.3% [5]. This number can be as high as 60-70% in critically ill COVID-19 patients with ARDS as the main symptom [8,9]. A recent pooled analysis of 331 COVID-19 patients receiving ECMO therapy suggested a CFR of 46% (95%CI: 34%–59%) [4]. The CFR found in our study was 55.6% (5/9), however, which may be biased by the small sample size. As a comparison, a previous study showed that ECMO support can lower CFR to 21% in patients with 2009 Influenza A (H1N1) ARDS [10].
As the last life-saving rescue strategy, ECMO is resourceintensive, highly specialized, high-priced, and extremely finite compared to the tremendous cases. Therefore, it is urgently needed to identify the potential factors that were associated with prognosis of an individual patient and facilitate the frontline health workers to optimize the use of limited medical resources. Some previous studies have explored the risk factors for critical and mortal COVID-19 patients. A cohort including 201 patients found that older age, neutrophilia, and organ and coagulation dysfunction were risk factors associated with the development of ARDS and progression from ARDS to death. A recent study found that severe CO2 retention and acidosis prior to ECMO indicated a poor prognosis among COVID-19 patients receiving ECMO support 9. Additionally, IL-6 was reported to be positively associated with risk of death in COVID-19 patients which received ECMO therapy [11,12]. In this study, our findings demonstrated that ABO blood group and changes of Pao2/Fio2, lactic acid, NLR, and CRP around ECMO initiation may be related to prognosis of ECMO support among COVID-19 patients with ARDS.
A very recent genome wide association study of severe COVID-19 published on the New England Journal of Medicine confirmed that the ABO blood-group system played key roles in the development of COVID-19 13. Blood group A was associated with a higher risk of acquiring COVID-19 than non-A blood groups, whereas, blood group O was associated with a lower risk than non-O blood groups [13]. Of the nine COVID-19 patients included in our study, four were blood group A, four were blood group B, and the other one was blood group AB. In consistent with the reported findings, we found that all of the four patients of blood group A died after ECMO support, however, only one of the five patients of blood group B/AB died after ECMO support. None of the patients had blood group O in our study, therefore, we were not able to evaluate the effect of blood group O on outcome of ECMO therapy
Pao2/Fio2 is an important index used to evaluate a patient’s pulmonary oxygenation and severity of ARDS [14]. Based on the values of Pao2/Fio2, ARDS is categorized into mild (200 mm Hg< Pao2/Fio2 <300 mm Hg), moderate (100 mm Hg< Pao2/Fio2 <200 mm Hg), and severe (Pao2/Fio2< 100 mm Hg) [15]. Our findings in this study clearly demonstrated that if Pao2/Fio2 increases to higher than 100 mm Hg in the first 48 hours of receiving ECMO, the patients may be survival to discharge. In other words, if Pao2/ Fio2 was still less than 100 mm Hg after 48-hour ECMO support, the patient generally had severely impaired pulmonary function that cannot be improved by ECMO support. Lactic acid is a metabolic product of anaerobic glycolysis and has been used widely as a marker of altered tissue perfusion in critically ill patients [16]. Elevated lactic acid level may reflect inadequate oxygen delivery [17] and is associated with higher mortality rate in critically ill patients [18]. Inconsistent with the above-mentioned findings on Pao2/Fio2, decreased plasma lactic acid level in the first 48 hours of ECMO therapy mean that the patients may have a higher probability to be survival to discharge eventually.
It has been reported that SARS-Cov may act on T lymphocytes and exacerbate a patient’s immune function [19]. COVID-19 is generally accompanied with a high incidence of lymphopenia. Lymphocyte has been thought as a potential indicator for critical illness of COVID-19 [20]. In this study, we found that increased lymphocyte count and decreased NLR in the first 48 hours of receiving ECMO support, which means that immune function was improved, was associated with favorable prognosis in COVID-19 patients with ARDS. CRP is a non-specific acute-phase biomarker of inflammation, infection, and tissue damage [21]. CRP facilitates clearance of pathogenic microorganisms invading the body through complement activation and enhanced phagocytosis [22]. CRP concentrations have been reported to be positively associated with lung lesions at the early stage of COVID-19 and the severity of COVID-19 pneumonia [23,24]. Our findings indicate that if a patient’s CRP level was decreasing during the 48 hours before ECMO support, then ECMO therapy may not benefit this patient much because the patient’s immune function may be in a stage of deterioration.
Some limitations should be kept in mind when we interpret these findings. The sample size is small though all of the ECMO cases in these two hospitals have been included in our study, which limited the extrapolation of our finding to general COVID-19 patients with ARDS. Thus, the clinical experience on ECMO the summarized in this study should be validated in further COVID-19 case cohort.
Conclusion
We reported a proportion of 44.4% (4/9) COVID-19 patients with ARDS receiving ECMO therapy were survival to discharge. Moreover, we found five factors which may facilitate the doctors in ICU to optimize the very finite ECMO resources: blood group A and a decreasing CRP level before ECMO initiation may be associated with mortality outcome; Pao2/Fio2 rising to higher than 150 mm Hg, increased lymphocyte count, and decreased lactic acid and NLR in the first 48 hours of ECMO support may be associated with survival outcome.
Supplementary Information
Supplementary information providing additional Table S1 in one Microsoft Word file. Additional file 1: Table S1. Changes of clinical characteristics of ECMO support in COVID-19 patients with ARDS.
Acknowledgement
The authors would like to express their sincere gratitude to all the participants who were involved in this study, and the research assistants for the support they provided. Without their co-operation this study would not have been successful.
Authors’ contribution
JL, XL, and BH conceived and designed the research. Material preparation, data collection and analysis were performed by JL, XL, BH, CBZ, LHL, CM, JMW, HGZ, JXL, PJ, RQY, CQL, ZJL, XZ, FZ and DD. The manuscript was drafted by JL and all authors read and approved the final manuscript.
Funding
This work was supported by the Tibet Autonomous Region Research Projects (No. XZ2018-01-GB-09 to X. Li), Guangdong Medical Scientific Research Foundation (A2017297), the Science and Technology Planning Project of Guangzhou, China (201804010335) and the High-level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJH2020020).
Availability of Data and Materials
Datasets are available on request.
Ethics Approval and Consent to Participate
This study was approved by the ethnic committees of GDPH, JZCH, and PHSZ. Written informed consent was obtained from all patients or their surrogates.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
References
- Guan WJ, Ni ZY, Hu Y, Wen hua Liang, Chun quan Ou, et al. (2020) Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine 382(18): 1708-1720.
- Richardson S, Hirsch JS, Narasimhan M (2020) Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. Jama 323(20): 2052-2059.
- Ranieri VM, Rubenfeld GD, Thompson BT, Niall D Ferguson, Ellen Caldwell, et al. (2012) Acute respiratory distress syndrome: The Berlin Definition. Jama 307(23): 2526-2533.
- Melhuish TM, Vlok R, Thang C, Judith Askew, Leigh White, et al. (2020) Outcomes of extracorporeal membrane oxygenation support for patients with COVID-19: A pooled analysis of 331 cases. The American journal of emergency medicine.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. Jama 323(11): 1061-1069.
- Mi MY, Matthay MA, Morris AH (2018) Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. The New England journal of medicine 379(9): 884-887.
- Mac Laren G, Fisher D, Brodie D (2020) Preparing for the Most Critically Ill Patients With COVID-19: The Potential Role of Extracorporeal Membrane Oxygenation. Jama 323(13): 1245-1246.
- Henry BM, Lippi G (2020) Poor survival with extracorporeal membrane oxygenation in acute respiratory distress syndrome (ARDS) due to coronavirus disease 2019 (COVID-19): Pooled analysis of early reports. Journal of critical care 58: 27-28.
- Yang X, Cai S, Luo Y, Zhu F, Hu M, et al. (2020) Extracorporeal Membrane Oxygenation for Coronavirus Disease 2019-Induced Acute Respiratory Distress Syndrome: A Multicenter Descriptive Study. Critical care medicine 48(9): 1289-1295.
- Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. (2009) Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. Jama 302(17): 1888-1895.
- Henry BM (2020) COVID-19, ECMO, and lymphopenia: A word of caution. The Lancet Respiratory medicine 8(4): e24.
- Mehta P, Mc Auley DF, Brown M, Sanchez E, Tattersall RS, et al. (2020) COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet (London, England) 395(10229): 1033-1034.
- Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. (2020) Genome wide Association Study of Severe Covid-19 with Respiratory Failure. The New England journal of medicine 383(16): 1522-1534.
- Des Prez K, Mc Neil JB, Wang C, Bastarache A, Shaver CM, et al. (2017) Oxygenation Saturation Index Predicts Clinical Outcomes in ARDS. Chest 152(6): 1151-1158.
- Fan E, Brodie D, Slutsky AS (2018) Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. Jama 319(7): 698-710.
- Vincent JL, De Backer D (2013) Circulatory shock. The New England journal of medicine 369(18): 1726-1734.
- Mungan İ, Kazancı D, Bektaş Ş, Ademoglu D, Turan S (2018) Does lactate clearance prognosticates outcomes in ECMO therapy: a retrospective observational study. BMC anesthesiology 18(1): 152.
- Vincent JL, Quintairos ESA, Couto L, Taccone FS (2016) The value of blood lactate kinetics in critically ill patients: a systematic review. Critical care (London, England) 20(1): 257.
- Liu WJ, Zhao M, Liu K, Xu K, Wong G, et al. (2017) T-cell immunity of SARS-CoV: Implications for vaccine development against MERS-CoV. Antiviral research 137: 82-92.
- Liu J, Liu Y, Xiang P, Pu L, Xiong H, et al. (2020) Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. Journal of translational medicine 18(1): 206.
- Pepys MB, Hirschfield GM (2003) C-reactive protein: A critical update. The Journal of clinical investigation 111(12): 1805-1812.
- Warusevitane A, Karunatilake D, Sim J, Smith C, Roffe C (2016) Early Diagnosis of Pneumonia in Severe Stroke: Clinical Features and the Diagnostic Role of C-Reactive Protein. PloS one 11(3): e0150269.
- Wang L (2020) C-reactive protein levels in the early stage of COVID-19. Medicine et maladies infectiousness 50(4): 332-334.
- Chen W, Zheng KI, Liu S, Yan Z, Xu C, et al. (2020) Plasma CRP level is positively associated with the severity of COVID-19. Annals of clinical microbiology and antimicrobials 19(1): 18.

 Research Article
Research Article