Abstract
Background: Adverse drug reaction is any noxious, unintended, and undesired effect of a drug which resulted from inadequate monitoring of therapy or inappropriate dosing. It may be unexpected, unknown and/or rare. Adverse drug reactions (ADRs) are an important cause of mortality and morbidity worldwide. In some case it is lifethreatening and can be major determinants of treatment outcomes. All healthcare professionals are encouraged to report ADR, but under-reporting remains a major drawback of spontaneous reporting. Therefore, this study aims to investigate the knowledge, attitudes, and practices of healthcare professionals towards ADR reporting and try to fill the information gap in the study area.
Objective: To assess knowledge, attitude, and practice of ADR reporting among health care professionals working at Public Hospitals in Harar Town Eastern Ethiopia 2020.
Methodology: Health facility based cross sectional study was conducted on 238 Health professional who are working in Public Hospitals of Harar Town Eastern Ethiopia. Sample allocates proportionately and study participant was selected by systematic random sampling method. Collected and checked data were entered into Epi Data software version 3.1 and analysis was done by SPSS version 21. Mean value were used to classify as good or poor knowledge, altitude and practice on ADR reporting. Finding was summarized and presented in forms of tables and statement.
Result: The overall prevalence of good knowledge, altitude and practice of ADR reporting was 42.9 %, 34.5 % and 39.9 % respectively. Majority 158 (66.4%) of study participant does not feel that there are adequately trained on ADR reporting. While 206 (86.6 %) and 208 (87.4%) of health professional agree that reporting drug safety is important for the public and health care system. One third of health professionals 74 (31.4%, P = 0.002) significantly reported that there had encountered ADR.
Conclusion and Recommendation: On this study majority respondent had poor knowledge, altitude and ADR Reporting practices. Therefore, Training provision, awareness creation, Strong and collaborative ADR reporting mechanisms, continuous monitoring and evaluation need to be established on each health institution.
Keywords: Adverse Drug Reaction; knowledge on ADR; ADR Reporting; Harar Town
Introduction
Adverse drug reaction is any noxious, unintended and undesired effect of a drug that occurs at doses used for prevention, diagnosis or treatment [1]. ADRs are defined as type A, type B, type C and type D [2]. Type A reaction (predictable) is related to dosage and is an extension to the normal pharmacology of the medication. Type B reaction (unpredictable) is unrelated to normal pharmacology. Type C reactions are associated with prolonged therapy e.g. analgesic nephropathy. Type D reactions are delayed reactions e.g. carcinogenesis and teratogenesis [3]. Most ADRs resulted from inadequate monitoring of therapy or inappropriate dosing [4]. All healthcare professionals including doctors, pharmacists, nurses and other healthcare professionals are encouraged to report ADR [5]. All healthcare providers have roles to play in maintaining a balance between a medicine’s benefits and risks [6].
Statement of the Problem
Adverse drug reactions (ADRs) are an important cause of mortality and morbidity worldwide [7]. ADRs may be unexpected, unknown and/or rare. They are in some case life- threatening and can be major determinants of treatment outcomes [8]. Underreporting remains a major draw-back of spontaneous reporting an estimated 6–10% of all ADRs are reported this can delay signal detection that will have negative impact on the public health [9,10]. Under-reporting is not the problems of developing countries only, for instance ADRs reporting rate in USA to be as low as 1 - 6% [11]. The situation is not different in Ethiopia where the level of ADR reporting is showed to be alarmingly very low Even though the spontaneous reporting system has been put in place as of 2002 and all health professionals are encouraged to report [12]. There for this study investigated the knowledge, attitudes and practices of healthcare professionals towards ADR reporting and try to fill the information gap.
Significance of the Study
Assessment of knowledge, attitude and practice of health care professionals has many importances it will evaluate the factors that could possibly affect adverse drug reaction reporting by observing the present system in place so that suggestions to improve reporting among health professionals can be offered. The result of the study will help in improving ADR reporting procedure by highlighting the drawbacks in the system. It will also serve as a base line data for other researchers
Objectives of the study
General Objective
To assess knowledge, attitude and practice of ADR reporting among health care professionals working at Public Hospitals in Harar Town Eastern Ethiopia 2020.
Specific Objective
1. To assess ADR Reporting knowledge of health care
professionals working at Public Hospitals in Harar Town
Eastern Ethiopia from February 7- February 25/2020 G.C.
2. To describe ADR Reporting altitudes of health care
professionals working at Public Hospitals in Harar Town
Eastern Ethiopia from February 7- February 25/2020 G.C.
3. To identify ADR Reporting practice of health care
professionals working at Public Hospitals in Harar Town
Eastern Ethiopia from February 7- February 25/2020 G.C.
Method and Materials
Study Area and Period
The study was conducted in Harar Town, which is one of the ten regional states of the Federal Democratic Republic of Ethiopia located in the Eastern part of the country at 526 km away from Addis Ababa, the capital city of Ethiopia. In the region, 3 governmental hospitals were found. Among those two, namely Jugel and Hiwot Fana specialized university hospital were selected by SRS and the study was conducted there from Feb 7 – 25, 2020 G.C
Study Design
Quantitative Institutional based cross-sectional study was utilized.
Population
Source Population: All medical doctors, Nurses, Pharmacists and Health officer working at Jugel and Hiwot Fana specialized university hospitals.
Study Population: Randomly selected Medical doctors, Nurses, Pharmacists and Health officer who are available on work during the study period
Inclusion and Exclusion Criteria
Inclusion Criteria: Professional who was volunteer to participate and gave consent.
Exclusion Criteria
1. Health professionals not willing to participate in the study
2. Health professionals who were absent at the time of the
study.
Sample Size Determination
Sample size was calculated for the three variables using of p - value for knowledge 76.9 %, altitude 93.8 % and reporting practice 64.6 % [13] by comparing the three-sample size the highest was taken which were 351. Sample was reduced by using sampling size estimation method and finite population correction formula and the total sample become 216. Then by adding 10% non-response rate, the final sample size was = 238.
Sampling Technique and Procedure
Two governmental Health institutions were selected by simple random sampling method. After obtaining the total number of professional (Nurse, Medical Doctors, Pharmacist and Health officers) of both hospital sample was proportionately allocated. Individual study subjects at each health facility were selected by systematic random sampling.
Variables
Dependent variable: KAP of ADR reporting Independent Variable: Age, Educational level, Sex, profession, year of experience, patient load.
Data Collection Tool and Method: Data collection tool was developed after review of literature and using previous studies. It contains question that was assess socio demographic, knowledge, attitude and ADR reporting practice. Face to face interview method was used to collect data.
Data Processing and Analysis: After data collection each questionnaire was checked its completeness, consistency on daily bases. Epi-Data version 3.1 and SPSS version 21 were used for data entry and analysis. Data were analyzed using descriptive statistics such as proportions, percentages, ratios. Measures of central tendency and measures of dispersion were done. Mean value was used to classify as good or poor knowledge, altitude and practice on ADR reporting and respondent who score mean and above the mean value were considered as good while the rest as poor.
Data Quality Assurance: To assure data quality it was be pretested on 5% the total sample in Harar Federal Police Hospital. Based on the results of pretesting necessary modification was made. Data collector and supervisor were trained for two day on objective of the study, method of data collection and discussed thoroughly on the tools and be for data collection they were allowed to fill the questionnaire and later discussion was made in all contents of the questioners and areas of difficulties were revised. The data was coded carefully in order to increase accuracy and quality of data.
Ethical Considerations:
Ethical clearance letter was obtained from Harar Health Science College Research Ethics Review Committee and it was submitted to the study organization and permission was secured from hospital CEO. All the participants were informed the purpose, advantages and disadvantages, there have the right to be involved or not as well as they can withdraw from the study any time they want. Informed consent was obtained. Confidentiality was maintained by avoiding names and other personal identification.Result
A total of 238 Health professional were included on the study which makes the response rate 100 %. The mean age of the study participants was 27.92 with ± 5.036 SD. Majority, 129 (54.2%) of the study participants were between 26 and 35 years. Regarding profession and year of experience majority 144 (60.5%) of the respondent had 0 - 4-year experience and 186 (78.2%) and Nurses with average mean monthly income of 4412.28 with ± 1623.21 SD ETB (Table 1).
Table 1: Socio- demographic characteristics of respondents working at Public Hospitals in Harar Town Eastern Ethiopia 2020 G.C.
Knowledge of Respondents on ADR reporting
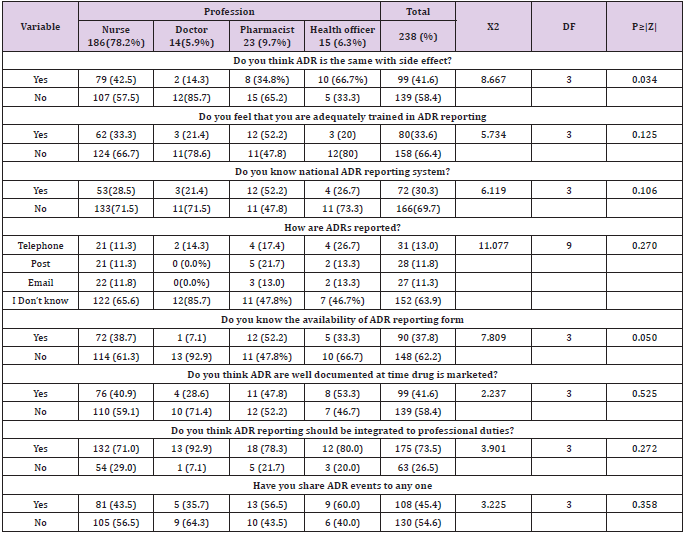
Among study participant 102 (42.9 %) had good knowledge while the rest 136 (57.1%) had not. Majority 139 (58.4%) of study participant replied that ADR is not the same with side effect and 158 (66.4%) study participant does not feel that there are adequately trained on ADR reporting. More than half of study participant 166 (69.7%) and 148 (62.2%) do not know the availability of national ADR reporting system and ADR reporting form. While 99 (41.6%) and 175 (73.5) respondents replied that ADR are not well documented at time drug is marketed and better ADR reporting should be integrated to professional duties. More than half of professional 130 (54.6%) doesn’t share ADR events to anyone (Table 2).
Table 2: Knowledge regarding adverse drug reaction reporting among health professional in Public Hospitals of Harar town, Eastern, Ethiopia 2020.
Altitude of Respondents on ADR reporting
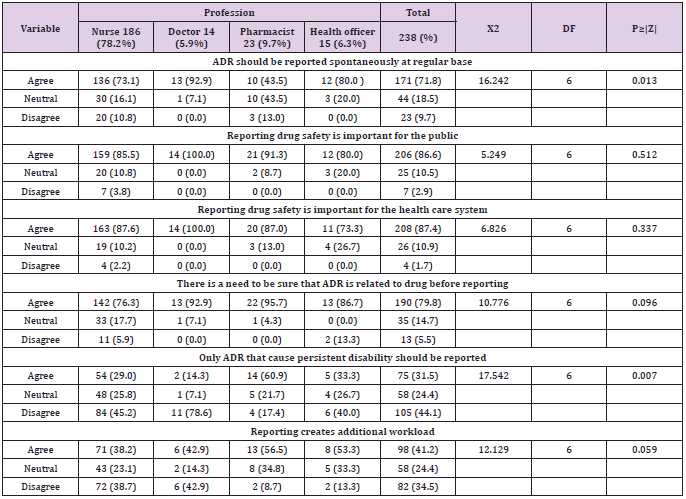
The overall prevalence of good altitude was 82 (34.5%) and majority 156 (65.5%) had poor altitude towards ARD Reporting. More than half 171 (71.8%) of respondents agree that ADR should be reported spontaneously at regular base. While 206 (86.6 %) and 208 (87.4%) of health professional agree that reporting drug safety is important for the public and health care system. Among study participants 190 (79.8%) and 105 (44.1%) of respondent agree and disagreed that there is a need to be sure that ADR is related to drug before reporting and also only ADR that cause persistent disability should be reported (Table 3).
Table 3: Altitude regarding adverse drug reaction reporting among health professional in Public hospitals of Harar town, Eastern, Ethiopia 2020.
Practice of the Respondents on ADR reporting
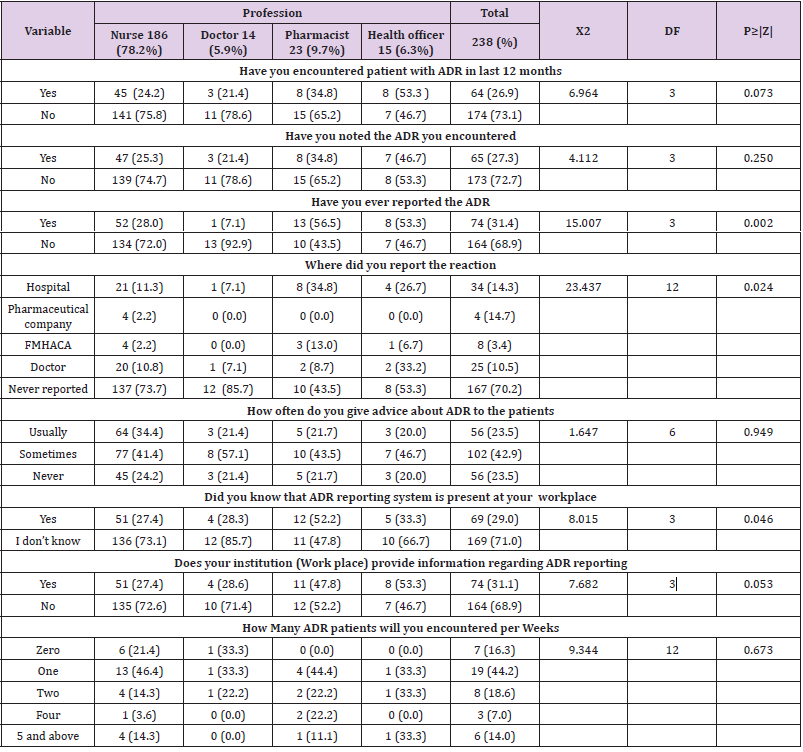
The overall prevalence of good ADR reporting practice was 95 (39.9%) while majority 143(60.1%) had poor ARD Reporting practice. One third 174 (73.1%) and 173 (72.7%) of health professional reported that they do not have encountered ADR and also do not noted the ADR their encounter. One third of health professionals 74 (31.4%, P = 0.002) significantly reported that ADR their encountered. And 102 (42.9) of health professional reported that they give advice sometimes on possible adverse effects of drugs they prescribed. Majority 196 (71.0%) and 164 (68.9%) of respondent reported that them don’t know the presence of ADR reporting system in their workplace and also their institution (Workplace) does not provide information regarding ADR reporting (Table 4).
Table 4: Practice regarding adverse drug reaction reporting among health professional in Public hospitals of Harar town, Eastern, Ethiopia 2020.
Discussion
The overall prevalence of good Knowledge, Altitude and practice on this study was low which was (42.9%), (34.5%) and (39.9%) respectively. The study was conducted in Harar town and 238 health professionals were participated, and majority were 186 (78.2%) Nurse followed by Pharmacist 23 (9.7%), health officer 15 (6.3%) and physicians 14 (5.9 %). In this study majority (58.4 %) of respondents replied that ADR is not the same with side effect which is lower than (76.9%) study conducted in Adam [13] this discrepancy might be due to difference in study area. In this study 80 (33.6 %) respondents felt that they are adequately trained in ADR reporting which was higher than study conducted in Adama (16.9%) [13]. In this study (69.7 %) of respondent do know the presence of national ADR of reporting system and (62.2%) also do not know the availability of ADR reporting form which was higher than study conducted in Adam (41.5 %) and (52.3%) respectively this difference showed that ADR reporting was not given emphasis and it doesn’t consider as a professional duties[13]. In this study (71.8%) of respondent agree that ADR should be reported spontaneously at regular base this finding in line (70.6 %) with study conducted in Pakistan [14]. Regarding the presence of ADR reporting system in our study (71%) of respondent did not know presence of ADR reporting system which was higher than study conducted in Pakistan (53.4 %) [14].This difference might be due to study setting variation. Regarding ADR training in this study (66.4%) feel that there are not adequately trained in ADR reporting which was lower than study conducted in (86.9%) Pakistan [13] and (83.1%) Adama [14] this difference might be due to variation in sample size and study population.
Conclusion and Recommendation Recommendations
On this study majority respondent had poor knowledge, Altitude and ADR Reporting Practices this may showed that ADR reporting would not give emphasis as a duty of health professionals and it was neglected. Even if ADR encounter during their practice on daily based, majority of professional does not know the presence of national ADR reporting system and the availability of ADR reporting form. Therefore, in order to improve ADR reporting Knowledge Altitudes and practice of health Professionals Training provision, awareness creation, Strong and collaborative ADR reporting mechanisms, continuous monitoring and evaluation need to be established on each health institution. Finally, health care tires need to give emphasis and established functional structure that strongly maintains ADR reporting activities.
References
- (2002) World Health Organization the Importance of Pharmacovigilance: Safety Monitoring of Medicinal Products. Geneva.
- Lisha Jenny John, Mohamed Arifulla, Jenny Cheriathu, Jayadevan Sreedharan (2012) Reporting of Adverse Drug Reactions: a study among Clinicians. Journal of Applied Pharmaceutical Science 2(6): 135-139.
- Santosh KC, Pramote Tragulpiankit, Sarun Gorsanan, I Ralph Edwards (2013) Attitudes among healthcare professionals to the reporting of adverse drug reactions in Nepal. BMC Pharmacology and Toxicology 14: 16.
- Fadare J Knowledge (2011) Attitude and Practice of Adverse Drug Reaction Reporting among Healthcare Workers in a Tertiary Centre in Northern Nigeria. Trop J Pharm Res 10(3): 236.
- Fadare J Knowledge (2011) Attitude and Practice of Adverse Drug Reaction Reporting among Healthcare Workers in a Tertiary Centre in Northern Nigeria. Trop J Pharm Res 10(3): 236.
- Zolezzi M, Parsotam N (2005) Adverse drug reaction reporting in New Zealand: implications for pharmacists. School of Pharmacy, University of Auckland, Auckland, New Zealand 1(3): 181-188.
- Rajesh A Kamtanel, V Jayawardhani (2012) Knowledge, attitude and perception of physicians towards ADR reporting: A pharmaco epidemiological study. Asian J pharm clin Res l5(l3): 210-214.
- (2006) World Health Organisation. The Safety of Medicines in Public Health Programmes: Pharmacovigilance and essential tool. The Uppsala Monitoring Centre. Geneva, Switzerland.
- Lisha Jenny John, Mohamed Arifulla, Jenny Cheriatu, Jayadevan Sreedharan (2012) Reporting of Adverse Drug Reactions: a study among Clinicians. Journal of Applied Pharmaceutial Science 2(6): 135-139.
- Lorna Hazell, Saad AW Shakir (2006) Under Reporting of Adverse Drug Reactions: A systematic Review. Drug Safety 29(5): 385-396.
- Chyka PA (2000) How many deaths occur annually from adverse drug reactions in the United States. American Journal of Medicine 109: 122-130.
- Ermias A, Gurmesa G, Mesfin M, Mengistu A (2011) Adverse Drug Reaction Monitoring in Ethiopia: Analysis of case reports, 2002-2007. Ethiopian Journal of Health Development 25(2): 168-173.
- Mohammed H, Bule B (2016) Knowledge, attitudes and practices of healthcare professionals towards adverse drug reaction reporting in Adama hospital medical college, east Shoa zone, Oromia regional state, Ethiopia. The Pharma Innovation Journal 5(7): 24-28.
- Zaka U, Ayesha Z, Farooq S (2018) Assessment of knowledge, attitude and practice of adverse drug reaction reporting among healthcare professionals in secondary and tertiary hospitals in the capital of Pakistan. Saudi Pharmaceutical Journal 26(4): 453-461.

 Research Article
Research Article