Abstract
Therapeutic Drug Monitoring (TDM) is an evidence-based practice consistent with
the assumption that pharmacological plasmatic concentrations correlate better with
clinical effects than prescribed doses of the used drugs. TDM has several indications in
treated psychiatry patients: comorbidities, suspected non-compliance, severe adverse
effects and tailored pharmacotherapy. Antidepressant Drugs (AD) are prescribed in
patients with Eating Disorders (ED) to treat depression or anxiety disorders associated
with ED or to reduce binge-eating behaviours. TDM may represent a valid tool in this
population, considering the limited efficacy of ED’s pharmacological treatment and
the high rate of adverse effects. Nineteen outpatients affected by ED with a Body Mass
Index (BMI) < 20 or > 30 kg/m2 treated with antidepressants agreed to participate in
this study. Participants were treated with Sertraline (N=5), Fluoxetine (N=6), Vortioxetine
(N=4), Citalopram (N=2), Escitalopram (N=1), Fluvoxamine (N=1). Oral fluid samples and
whole blood dried microsamples by finger puncture using VAMS (Volumetric Absorptive
Microsampling) technique were obtained from patients. Sociodemographic and clinical
information were also collected.
Preliminary results by our pilot study show a correlation between plasmatic and
salivary concentrations only for Vortioxetine but not for other examined antidepressants.
Additionally, plasmatic concentrations of all examined antidepressants are constant
for extreme BMI when dose-corrected. By considering these preliminary data, we are
confident that further studies characterized by the expansion of the sample size will allow
us to outline that TDM may represent a valid tool in order to better explain the limited
efficacy of AD in patients with starvation state or obesity.
Keywords:Therapeutic Drug Monitoring; Eating Disorders; Antidepressant Drugs; Plasmatic concentrations
Abbreviations: ED: Eating Disorders; BED: Binge-Eating Disorder; BN: Bulimia Nervosa; AD: Antidepressant Drugs; TDM: Therapeutic Drug Monitoring; SSRI: Selective Serotonin Reuptake Inhibitor
Introduction
Patients with Eating Disorders (ED) including, among others,
Anorexia Nervosa (AN), Bulimia Nervosa (BN) and Binge-Eating
Disorder (BED) suffer from a persistent disturbance of eatingrelated
behavior that results in the altered consumption or
absorption of food and that significantly impairs physical health
and/or psychosocial functioning [1]. The latest guidelines regarding
ED [2] recommend a multidisciplinary intervention, including
psychological treatment, dietary advice and medication. Despite
the body of research on ED psychopharmacologic treatment has
increased lately, there remains a paucity of appropriately sized
Randomized Controlled Trials (RCTs) and a great need of further
studies on pharmacotherapy agents in ED [3], and especially in AN [4-6]. Even if many medications have been used in patients
affected by AN, outcomes are generally poor. The lack of response
to medications may be the result of the complicated physiological
state of starvation [5].
Fluoxetine, for example, which is the only Selective Serotonin
Reuptake Inhibitor (SSRI) approved to treat BN [7], does not
increase patients’ Body Mass Index (BMI) and does not show any
significant psychopathological improvement in patients with
AN [5]. Current treatment guidelines do not recommend the use
of SSRIs as unique therapeutic approach for AN due to limited
efficacy [2,8] and increased risk of adverse effects [9]: there is a
lack of evidence for the use of SSRIs in underweight patients for
specific AN-related symptoms [8], although the use of SSRIs
may help in relapse prevention and improvement of psychiatric
comorbidities (eg. depression, anxiety and OCD) [6,8], above all
when weight is restored. Nevertheless, SSRIs are actually the most
common category of psychotropic drugs prescribed in patients
with ED [10,11], even during acute phases, explaining the so-called
“research-practice gap” (patients not always receiving treatments
rooted in scientific evidence) [12].
It was suggested that SSRIs have decreased efficacy in anorexic
patients with low BMI due to starvation-related biochemical
changes in the brain [13] and because of inadequate concentration
of nutrients, which are necessary for serotonin metabolism [14].
Nevertheless, nutritional supplements containing tryptophan
and essential fatty acids do not seem to increase SSRIs efficacy in
underweight patients with AN [15]: the potential reason remains
uncertain, but the latest genetic findings suggest that ED may not
only be seen as psychiatric, but also as metabolic and immune
disorders [6,16]. In a study on patients treated by Risperidone,
Paulzen M. and colleagues suggested that extreme low and high
BMIs modify psychotropic drugs’ metabolism, in particular
pharmacokinetic parameters [17]: obese patients showed a higher
plasma concentrations of the active metabolite of Risperidone
when compared with cachectic patients; authors speculate that
this pharmacological behaviour is due to cytochrome P450
activity alterations or differences in P-glycoprotein function. Other
studies showed negative correlation between BMI and plasma
concentration of psychotropic drugs and/or their metabolites
[18,19] and these results are explained by the authors as linked
to different distribution volumes for lipophilic substances (larger
distribution volume in obese subjects leading to inadequate plasma
levels and smaller therapeutic efficacy) [19]. Unterecker et al.
instead point out the absence of any relationship between body
weight and plasma concentrations of antidepressants, highlighting
the need for further exploration studies [20]. Vortioxetine is an
antidepressant with multimodal activity currently approved for
the treatment of major depressive disorder that is metabolized
by cytochrome P450 enzymes and subsequently by uridine
diphosphate glucuronosyltransferase. Several evidences from
current literature do not show clinically relevant differences
in vortioxetine exposure by sex, age, race, body size, and renal
or hepatic function. Dose adjustment is only recommended for
cytochrome P450 2D6 poor metabolizers and when it is associated
with bupropion, a strong cytochrome P450 2D6 inhibitor, and
rifampin, a broad cytochrome P450 inducer.
The simultaneous administration of other drugs does
not affect vortioxetine exposure or safety profile. Moreover,
pharmacodynamic findings demonstrate that vortioxetine achieves
high levels of serotonin transporter occupancy in relevant brain
areas and modified abnormal resting state networks in the brain
over the therapeutic dose range. Overall, vortioxetine can be
administered without major dose adjustments [21]. Considering
the altered physical and metabolic condition of patients with
extremely low and high BMIs, we aimed to investigate the
usefulness and feasibility of minimally-invasive and miniaturised
biosampling techniques used for Therapeutic Drug Monitoring
(TDM) of psychiatric Central Nervous System (CNS) drugs [22,23]
in outpatients affected by ED. TDM of SSRIs has already several
approved indications such as lack of clinical response despite
adequate doses, adverse effects using recommended doses, and
drugs’ prescription in patients with pharmacokinetically relevant
comorbidities (eg. extremely high or low BMI) [24,25], to obtain
better therapy optimization and personalization [25,26]. Moreover,
TDM can also lead to reduced healthcare expenses, due to the
possibility of better efficacy, increased patient compliance and
enhanced safety, leading to a reduction in hospitalizations due to
unwanted effects or therapy ineffectiveness [27-30].
In this pilot study we have described the use of Volumetric
Absorptive Microsampling (VAMS) in the management of
pharmacological treatment of ED patients in outpatient setting
in order to analyse any pharmacokinetic differences of the main
antidepressants in use in patients with very varied ED and BMI. A
secondary goal is to test for the first time the applicability of the
VAMS technique for the analysis of antidepressants in whole blood
and Oral Fluid (OF) for the purposes of TDM. The main aim could
help us to better describe and understand the relationship between
the BMI and biological fluid concentrations of antidepressant drugs
together with their metabolites in these cohort of patient. In order
to do that, we have carried out an analytical workflow based on
VAMS of both whole blood and OF, followed by microextraction by
packed sorbent (MEPS) [23] and Liquid Chromatographic (HPLC)
analysis with spectrophotometric (UV) and spectrofluorimetric
(FL) detection.
Experimental Procedures
Participant’s Enrolment
From January 2019 to May 2019, a total of 19 patients consecutively evaluated at the outpatient Unit for Eating Disorders of Bologna University (Northern Italy) were enrolled in the present study. This outpatient unit for ED is an academic clinic specialized in the diagnosis and treatment of ED in adult individuals (≥18 years old). Patients were asked to participate in the study whether they met the following inclusion criteria:
a) a diagnosis of Eating Disorder according to DSM-5
criteria(American Psychiatric Association, 2013),
b) BMI < 20 kg/m2 or > 30 kg/m2,
c) age ≥ 18 years and ≤ 30 years,
d) pharmacological treatment (SSRIs or vortioxetine).
Exclusion criteria were the presence of other several mental disorders, in particular schizophrenia, substance abuse and bipolar disorder.
Patient’s Assessment
The evaluation protocol at the outpatient unit for ED consisted of three different meetings with a psychiatrist. In the first meeting, socio-demographic and clinical data were collected from the patients and from their medical records; during the second consultation, we filled in a psychopathological assessment including some specific tools for ED; finally, during the third meeting the psychiatrist shared the diagnostic conclusions with the patient and suggested the type of treatment. In case of eligibility for the present study, a fourth meeting was scheduled for the biosampling session. During this last consultation, additional information were collected by the physician, including the type of antidepressant drug, dose, last intake, first prescription of the treatment and compliance.
Ethical Issue
The present study was approved by the local Ethical Committee (Comitato Etico Indipendente di Area Vasta Emilia Centro, code: CE18135) and all recruited patients signed the informed consent. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975 and its most recent revision.
Biosampling
Patients participating in the present study were scheduled
to be visited at the ED outpatient clinic in the morning between
10 a.m. and 12 a.m. Patients were informed neither to drink nor
eat in the 30 minutes before the consultation. The intervention
consisted in the microsampling of whole blood in dried form
using VAMS approach and the collection of OF after reaching the
steady-state for the antidepressant drug (fourth meeting). In fact,
TDM procedures must be carried out under steady-state condition,
which is reached within a time of 4-5 half-lives [22]. For different
SSRIs and vortioxetine, at least 14 days of treatment are necessary
to get to steady-state conditions.
Blood drops were obtained by minimally invasive finger
pricking by means of a sterile, disposable needle, while whole blood biosampling was carried on using specific devices based on
VAMS technology. VAMS devices are particularly easy to handle
and facilitate the contact between the polymeric head of the device
(“tip”) and blood drops. In the present study, three 20-μL tips were
obtained for each patient, representing identical sample replicates.
Simultaneously, OF was collected by spitting in a dedicated
microtube. VAMS devices were then stored in a tightly closed
container and in the dark at room temperature. Storage clamshells
provided as part as VAMS packages were used in the present
study before the analysis process, which was carried out by the
research group of Pharmaco-Toxicological Analysis (Department
of Pharmacy and Biotechnology, FaBiT – University of Bologna).
On the other hand, OF was stored at the temperature of 0°C before
transportation to the Analysis Laboratory. Analysis were carried
out by means of fully validated analytical methodologies.
Materials
Duloxetine hydrochloride (used as the internal standard IS1 for HPLC-UV) and Venlafaxine hydrochloride (used as IS for HPLC-FL), pure powders (all >99% purity); acetonitrile, methanol and dichloromethane (for HPLC, purity: > 99.9%), monobasic potassium phosphate, Triethylamine (TEA), phosphoric acid, sodium carbonate and potassium hydroxide (all pure for analysis) were purchased from Sigma Aldrich Italy (Milan, Italy). Clotiapine (used as IS2 for HPLC-UV) pure powders were purchased from LGC Standards (Teddington, Middlesex, UK). Ultrapure water (18.2 MΩ cm) was obtained by means of a Milli-Q apparatus from Millipore (Milford, MA, USA). ISS stock solutions (1 mg/mL) were prepared by dissolving suitable amounts of pure powders in methanol and kept at -20°C when not in use; the corresponding standard solutions were prepared daily by dilution with the HPLC mobile phase. All solutions were stored protected from light in amber glass vials from Phenomenex (Torrance, CA, USA).
Hplc-Uv- Fl Instrumentation And Conditions
HPLC-UV- FL analysis was performed on a Waters Corporation (Milford, MA, USA) Alliance e2695 chromatographic system with autosampler coupled to a Waters 2998 photo diode array detector and a Jasco FP-2020 spectrofluorometric detector, connected in series. Separations were obtained on a Waters SunFire C18 column (100 x 3.0 mm, 3.5 μm) maintained at room temperature and equipped with a guard column. The mobile phase was a mixture of 33 mM, pH 3.0 aqueous phosphate buffer containing 0.3% TEA (solvent A) and acetonitrile (solvent B), flowing at a constant rate of 1.0 mL/min under gradient conditions. Gradient composition was: 0.0-3.0 min, constant 20% A; 3.1-4.0 min, linear 20%-35% A gradient; 4.1-6.5 min, constant 35% A; 6.6-7.5 min, linear 35%- 55% A gradient; 7.6-14.5 min, constant 55% A; 14.6-15.5 linear 55%-20% A gradient, 15.6-17.0 constant 20% A to re-equilibrate the column. Both solvents were filtered on a polyamide filter (47 mm dimeter, 0.2 μm) and degassed by ultrasonication. Injection volume was 20 μL. Sertraline (SRT), Norsertraline (NSR) and Vortioxetine (VTX) were detected by UV at 225 nm; Fluoxetine (FLX), Citalopram (CTP), Norfluoxetine (NFL), Dextcitalopram DCT and Diethyldithiocarbamate (DDC) were detected by fluorescence at λem = 235 nm, λexc = 300 nm.
Blood- and OF-VAMS Sampling and Pretreatment
For patient sampling, IS spiking was carried out on the VAMS
tip by automatic pipetting before the sampling; the tip was then
left to dry for 2 h at RT before use. Mitra® VAMS microsamplers
(20 μL) were provided by Neoteryx (Torrance, CA, USA). A VAMS
microsampler includes a polypropylene handle (about 4 cm long)
topped with a small tip (about 2-mm diameter) of a proprietary
polymeric porous material. B-VAMS. The finger prick site was
wiped with skin cleansing swabs and dried. Then a disposable
sterile lancet was used to prick the fingertip and a blood droplet
was allowed to form. Each VAMS tip was held at a 45° angle to the
surface of the blood droplet, taking care not to touch the skin. The
VAMS devices were held in this position until the whole tips were
visible filled with blood (around 5 seconds). The samplers were
then transferred to the dedicated clamshells, in order to avoid
contact with any surface and left to dry at Room Temperature (RT)
for 1 hour.
VAMS microsamples were thus obtained. Clamshells were
stored and transported at RT in sealed polyethylene bags
containing desiccant until pretreatment and analysis. For sample
pretreatment, the microsampler tip was detached from the handle
and subjected Ultrasound-Assisted Extraction (UAE) for 20 min
in 1 mL of methanol. The resulting solution was quantitatively
transferred into a different vial and brought to dryness in a
centrifugal evaporator. After re-dissolving with 100 μL of HPLC
mobile phase, the solution was subjected to Microextraction by
Packed Sorbent (MEPS) pretreatment in an SGE Analytical Science
(Melbourne, VIC, Australia) C2 barrel-and-needle (BIN) assembly
set up in an SGE eVol XR digital analytical syringe apparatus. The
BIN was activated by drawing and discharging 100 μL of methanol
3 times and conditioned with 100 μL of water 3 times. The sample
was loaded onto the BIN with 10 draw/discharge cycles at a 5 μL/s
speed; the BIN was then washed twice with 100 μL of water and
100 μL of 10 mM, pH 9.0 carbonate buffer / methanol (90/10, V/V)
mixture at 20 μL/s. The analyte and the ISs were eluted three times
with 200 μL of methanol at 5 μL/s (three cycles). After merging the
three eluates, they were brought to dryness, re-dissolved in 100 μL
of HPLC mobile phase and analysed by HPLC-UV-FL. OF-VAMS. OF
(1 mL) aliquots were centrifuged for 5 min at 6500 x g, then VAMS
samplers were used by touching the OF sample surface with the tip
and held in position for 10 seconds. Then, the same drying, storage,
pretreatment and analysis workflow as B-VAMS was carried out.
Statistical Analysis
All the statistical analysis were performed using SPSS IBM V.24.0 (statistical Package for Social Science). Other analysis (i.e. Linear Regression) will be performed in further studies when the sample size will be compatible with statistical power.
Results
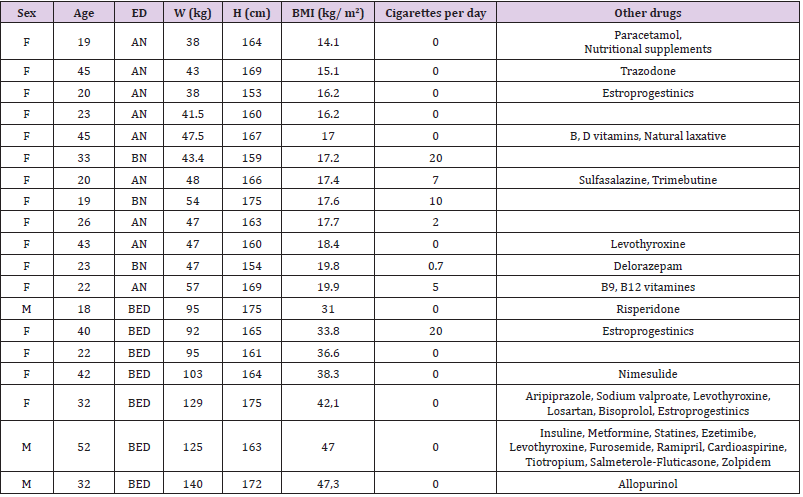
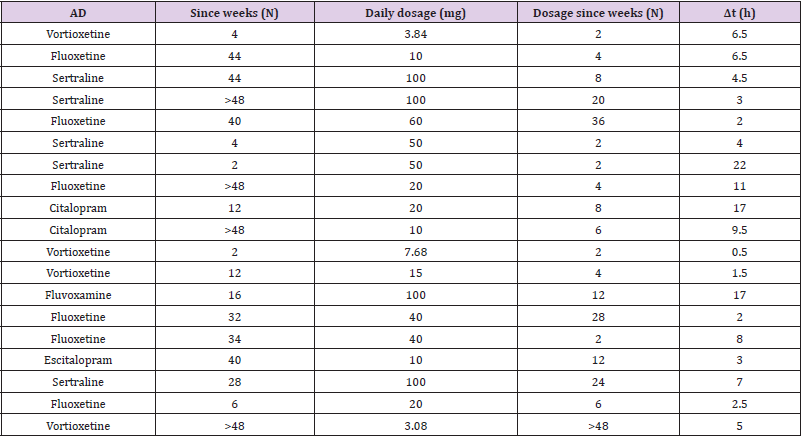
As reported in Table 1, 84.21% of enrolled patients are female. 47% of patients are affected by Anorexia Nervosa (AN), while 15% were diagnosed with bulimia nervosa and 38% with Binge Eating Disorder (BED). Median age of patients enrolled is 26 years, with a minimum age of 19 and a maximum of 52 years. Median weight is instead of 48 kg, with a minimum value of 38 kg and a maximum of 140 kg. Median BMI is 18.4 kg/m2 (underweight), with a minimum BMI of 14.1 kg/m2 (severe thinness) in a patient with AN and maximum BMI of 47.3 kg/m2 (severe obesity) in a patient with BED. As reported in Table 2, in order to treat psychiatric comorbidities or specific psychopathological issues of ED, we have treated our patients with one or more (1 patient) antidepressant drugs. 26.32% of patients is treated with vortioxetine (to treat major depression comorbidity in two patients with AN, one patient with BN and two patients with BED); 31.58% of patients take fluoxetine (in 2 patients with AN to treat depressive symptoms, in 1 patient with BN and 3 patients with BED to reduce binge eating behaviour); 26.32% of patients is treated with sertraline (to treat anxiety-depressive symptoms in 3 patients with AN, 1 patient with BN and 1 patient with BED); the 10.53% of patients is treated with Citalopram (in two patients with AN to treat anxiety symptoms); finally, one patient is treated with Escitalopram and one patient with Fluvoxamine (to treat the concomitant obsessive-compulsive disorder in comorbidity with BED).
Biological sampling was performed in all patients after reaching
steady state. The average time calculated between the start of
treatment (Time 0) and the biological sampling is 1.5 months,
with a minimum time of 2 weeks for sertraline, vortioxetine and
fluoxetine and a maximum time of 24 months for vortioxetine. This
duration could correlate with the amount of the drugs and their
metabolites that may have stored in the body, on the basis of their
pharmacokinetic characteristics. As shown in Table 2, Vortioxetine
has been prescribed in our population at a median dosage of 7.5
mg/day, while the median of the sertraline dosage is 100 mg/
day. Fluoxetine is prescribed at a median dosage of 30 mg/day.
Citalopram and escitalopram were both prescribed at a daily dose
of 10 mg, Fluvoxamine at 100 mg. The time, expressed in hours,
between the last intake of the drug and the collection of the biosamples
was variable (median of 5 hours, minimum value of 30
minutes and maximum of 22 hours).
In our sample, 36.8% of patients are smokers, while no patient
shows alcohol abuse. The information regarding the additional
drugs was taken, noted with the dosage and time of intake, and sent
to the analysis laboratory, so that any drug interactions potentially
altering the results of blood and OF concentrations can be taken
into account. In Table 1, additional drugs taken by patients are
shown as an indication and simplified. 47% of enrolled patients
take other drugs daily. This high rate recall the importance and the frequency of psychiatric and/or medical comorbidities in the cohort
of patients affected by ED and reinforce the rationale of this
study, i.e. the use of TDM in this specific population (Table 3). The
most informative results were obtained from four patients treated
with Vortioxetine. The first one is a 19 years old patient, female,
suffering by AN with BMI of 14.1 kg/m2. She has been treated with
Vortioxetine 5 mg/die for a month. The second one is a 22-year-old
female patient diagnosed with AN (BMI=19.9 kg/m2), treated with
15 mg/die of Vortioxetine. The third patient is a 23-year-old female
subject affected by BN (BMI=19.8 kg/m2), treated with Vortioxetine
at a dosage of 15 mg/die. Finally, a 32-year-old male patient affected
by BED (BMI=47.3 kg/m2) has been taking 4 mg/day of vortioxetine
for a long time. The collection of bio-samples took place between 30
minutes and 6.5 hours after the last intake of the drug, considering
that vortioxetine reaches the maximum whole blood concentration
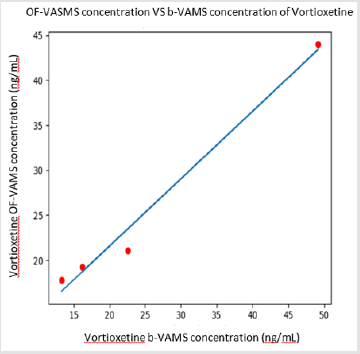
between 7 and 11 hours after the intake. The ratio between the
whole blood and salivary concentrations of Vortioxetine in the examined
patients is close to the unit as showed in Figure 1. Further
studies will allow us to expand the sample in order to quantify this
trend and to proceed with statistical analysis with the aim of determining
the statistical significance, supporting the potential use of
OF despite to the blood sample for TDM of Vortioxetine.
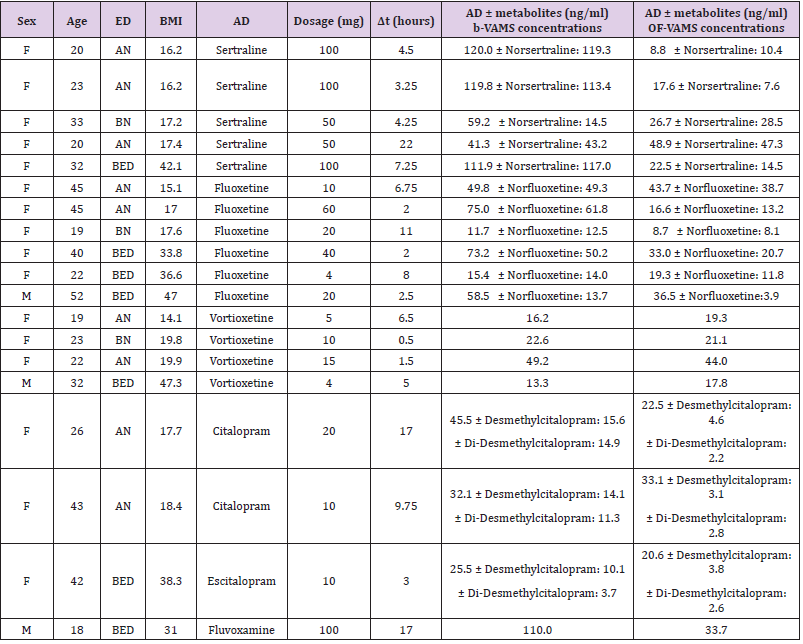
Table 3: ordered by type of prescribed antidepressant and increasing BMI, shows the concentrations obtained from b-VAMS and OF-VAMS analysis, highlighting levels of the drug and any active metabolites.
Considering a linear kinetic of Vortioxetine if we compare the corrected whole blood concentration of the drug for a dose of 5 mg/day to BMI, it emerges a higher ratio between whole blood Vortioxetine values and BMI in the patient with lower BMI (14.1 kg/ m2) than that with the highest BMI (47.3 kg/m2). The ratios are 1.15 and 0.35. Therefore, considering the very similar dose prescribed to the two patients with extreme BMI, we obtain in the underweight patient a whole blood concentration of Vortioxetine corrected for a dose comparable to that of the obese patient (16.2 ng / ml against 16.62 ng/ml respectively). Furthermore, in underweight patients, the free component of the drug may increase due to the reduced amount of albumin and circulating proteins available to bind the drug. The mean values of maximum whole blood concentration of vortioxetine obtained after multiple administrations of 5, 10 or 20 mg/day are typically between 9 and 33 ng/ml, according to the literature (Banca Dati Farmaci dell’AIFA). Taking into consideration that the interval between the intake of the drug and the evaluation of the whole blood concentration could differ significantly, we note that the patient with AN (BMI 19.9 kg/m2) has whole blood drug levels higher than the standard therapeutic interval (49.2 ng / ml), in the specific case the collection had taken place 5 hours after the last intake of the drug when the blood concentration has not yet reached the maximum level (Tmax between 7 and 11 hours after taking ). The data of the high blood concentration of Vortioxetine per oral dose in the dosage range appears significant due to fact that it does not appear to be attributable to pharmacodynamic or pharmacokinetic interactions of the molecule (the patient does not take other drugs or substances).
Discussion
The main goal of this study is to highlight a possible relationship between the whole blood and salivary concentration of antidepressants and their active metabolites using minimally invasive TDM methods such as VAMS strategy in a specific population affected by ED. This subset of psychiatric patients is extremely interesting in terms of correlation of peripheral concentration of drugs with oral dosage, because patients with ED have an important variability in terms of BMI. It is well known that BMI could affect clinical response to antidepressant drugs [31,32]. TDM can give us correct information on the concentration of active metabolites of antidepressants in peripheral matrices, net of the effect of liver metabolism and BMI on the pharmacokinetics of psychotropic drugs.
VAMS technology is currently a topic of enormous interest for the bioanalytical community and is the subject of numerous studies that have grown exponentially in the last two years [23,33-36]. VAMS devices were initially marketed for use on whole blood only; this method based on VAMS devices is applied also to the OF for the quantitative analysis of synthetic cathinones [36]. The aim of the present study, evaluating the concentration of antidepressants through VAMS devices both on whole blood and on OF, is to:
a) examine the relationship between the concentration of
drugs (above all Vortioxetine) and any active metabolites in the
two biological matrices and oral dosages of the same drugs;
b) analyze any pharmacokinetic differences of the main
antidepressants in use in patients with very varied ED and BMI.
Current findings in the literature concerning the correlation between whole blood and OF concentrations of psychotropic drugs outline information characterized by some limits. Often the data are fragmented, take into account very limited populations, or do not take into account the active metabolites of the drugs: some positive correlations have been highlighted between the whole blood and salivary concentration of monohydroxycarbamazepine [37], carbamazepine, phenytoin and phenobarbital [6,38]. For amitriptyline, nortriptyline and valproic acid, however, the correlation shown was not significant [6,38,39]. For a long time the concentration of drugs in OF has been considered as a reflection of the free component of the drug in the blood, which for many psychotropic drugs is only 10% or less of the total concentration; however, the distribution of drugs between blood and OF largely depends on the pH, which increases when the secretion is stimulated. OF is considered as an interesting and advantageous matrix for TDM studies, in particular due to the non-invasiveness, but data available so far in literature show contrasting results and further studies are necessary to validate the use of OF as a matrix for the TDM of antidepressant drugs [6,40]. On the other hand, according to the current literature [32], high BMI may also affect treatment response: Khan et al., [32] have found obesity to be associated with a less vigorous treatment response, with an effect more pronounced in males. Moreover, higher BMI was associated with a slower clinical response in the Munich Antidepressant Response Study (MARS) [41]. Finally, in the Genome Based Therapeutic Drugs for Depression (GENDEP) study, obesity was associated with a poorer response to nortriptyline in men and women, and a poorer response to escitalopram in women [19].
Several Proof-Of-Concept studies can be found in literature,
demonstrating the usefulness of VAMS devices for various
compounds and purposes, including the analysis of caffeine,
paraxantine, steroid compounds, 5-methyltetrahydrofolic acid, hydroxyurea, natural and synthetic cannabinoids, cocaine and
metabolites, oxycodone and metabolites, asenapine enantiomers
and iron isotopes [35,42-48]. To date, there are no studies on
reliability of VAMS technique for the analysis of antidepressant
concentrations, neither on blood nor on OF. In this context, this
study may be considered as a true innovative pilot study for the
usefulness and reliability of VAMS strategy within the TDM of
psychotropic drugs, above all in patients with a wide range of BMI
and pharmacokinetic characteristics.
In this context, our pilot study provides data about application
of VAMS for OF analysis, showing a good relationship between
whole blood and salivary concentrations for Vortioxetine only.
The ability to outline the usefulness of VAMS on OF may have been
hindered by the relatively small sample size. Future work should
seek to enrich the patient sample and to standardise the time of data
collecting and the last drug intake. The second macro-objective of
the study concerns the evaluation of the pharmacokinetic behavior
of antidepressant drugs in patients with extreme BMI (less than 20
kg/m2 and greater than 30 kg/m2). As explained in the introduction,
the role of body weight and BMI on the distribution and therefore
on the effectiveness of psychotropic drugs is particularly ambiguous
and relatively little debated in the literature; the results of the few
published studies are often incomplete and/or conflicting. The
present TDM study of antidepressant drugs in patients with ED and
therefore abnormal BMI allows us to:
a) Test in clinical practice the use of TDM in the
pharmacological treatment of patients with abnormal body
weight such as ED patients;
b) Contribute to the complex analysis of the factors that
modulate the clinical efficacy of psychotropic drugs through
a mainly pharmacokinetic perspective in a patient population
particularly resistant to drug treatment.
In relation to point 1., we can state that TDM is characterized as a potential Precision Medicine tool, capable of reducing the Trialand- Error approach and of favoring the therapist’s choices in an Evidence-Based prospective above all in outpatient setting where the patients have a low body weight and show ED symptomatology with several psychopathological dimension symptoms. According to the guidelines, and even more so with respect to such fragile patients, often with physical comorbidities and with very low BMI, the psychiatrist’s habit is to prescribe minimal doses of antidepressant drug (SSRIs, vortioxetine, etc.) and to begin a progressive and slow titration of the dosages. Often, the patient is not affected by any benefit or only reports the appearance of undesirable effects. When the medium-low dose drug treatment is not effective, the therapist must empirically evaluate whether to increase the dosage or to change the type of psychiatric drug. The clinician’s resistance in prescribing the highest dosages allowed for a given antidepressant to patients with very reduced BMI recalls the general principles of pharmacology according to which low distribution volumes (AN patients) will correspond to higher blood concentrations for a given dose of drug in ratio to patients with BMI in the normal range, population in which blood concentrations are studied with reference to a given oral dose.
In outpatient setting, a fortuitous trial-and-error process is used to try to reach the optimal dose for the specific patient [26,49]. This process does not favor the therapeutic alliance and explains the difficulties of treatment and healing. Instead, the psychiatrist can make targeted decisions relying on non-invasive and timely TDM methods: firstly, to rule out that the patient is not taking the drug at all or that he is taking it incorrectly (as discussed in the introduction part, adherence to pharmacological prescriptions are often scarce in this type of patients particularly ambivalent towards the process of change towards healing); secondly, to increase the dosage when whole blood concentrations of the antidepressant are too low or to change the type of drug if whole blood concentrations are approaching the upper limit of the therapeutic window, without showing the clinical expected results. In patients with high BMI we can also evaluate the whole blood concentration of the drug and therefore modify the daily dose or the molecule according to the pharmacokinetic characteristics of the individual patient.
In consideration of point 2., however, it is necessary to consider the pharmacokinetic variables and the absorption, distribution and elimination characteristics of the antidepressant drugs considered in this study, described in the introduction, except for vortioxetine, chosen in this study, for the peculiar pharmacokinetic profile, as well as for the uniqueness of the pharmacodynamic profile [21]. Overall, the pharmacokinetic factors to be taken into account when describing the concentrations of antidepressants in blood and OF of such patients with particular physical characteristics are numerous and intricate. Ideally, the chemical-physical properties of the drug, its absorption, first pass metabolism, bioavailability, protein binding, distribution in abnormal body volumes (in association with the study of body composition), should be taken into account. The fat content and visceral obesity could explain some differences in the response to antidepressant drugs [19], the passage at the level of the blood-brain barrier to reach the target sites where the action is performed from a pharmacodynamic point of view, as well as the mechanisms of final metabolism and excretion. Added to this is the role of pharmacogenetics and the inter-individual variability, characterized by the different levels of cytochrome activity. Among other things, this activity is modified by obesity and the type of diet, as shown by various studies [50-52].
Our results, in line with recent literature data [20], support the absence of a significant influence of BMI on drug whole blood concentrations in relation to the oral posology taken. This data partially contrasts with the pharmacokinetic behavior generally expected by the clinician on the basis of the fundamental principles of pharmacology: in a large distribution volume (patient with BED) the whole blood concentration of the drug will be lower than in a patient with a very poor volume distribution (patient with AN). Considering that routine blood tests are systematically prescribed during the taking in charge at the Unit for the Study and Assistance of the DCA and that patients are followed in an outpatient setting without the needing of hospitalization, it is possible to affirm with relative safety that the patients enrolled in the present study do not show significant alterations in renal and/or hepatic function, although the latter have not been thoroughly evaluated with specialized tests [53-55].
This pilot study reports numerous limitations for logistical reasons, having been conducted in a “real world” setting with all the difficulties associated with the organization of the collection of samples in an outpatient setting, the variability and inconstancy in taking the drug by patients, the timing of the withdrawal, difficult to agree with patients due to their unavailability. The limits also include devices and analysis procedures cost, and the low number and heterogeneity of the sample under study: the great variability of the BMI included, of the drugs taken and the medical comorbidities limit the possibility of examining correctly the relationship between drug concentrations in the matrices examined in such a small sample of patients. Our aim is to perform further studies in order to obtain a enriched sample size, a better standardization to reduce Δt variations among the sample of patients, the number and the differentiation of the various antidepressants in favor of an expansion of the cohort of patients who take Vortioxetine due to demonstrated tendency to provide a relationship that approximates the unity between salivary and whole blood concentrations.
Furthermore, the present study lacks correlations between clinical outcome and plasmatic antidepressant concentrations: in further studies it would be useful to consider using more specific psychiatric scales to accurately correlate efficacy and adverse effects to plasmatic antidepressant ranges. The applicability of effectiveness and minimally invasive TDM techniques opens the doors to the use of these tools in populations that are even larger and not spared by the appearance of ED, such as the pediatric one. The evaluation of whole blood and salivary concentrations of antidepressants in underweight or obese patients with ED allows us to formulate hypotheses and pharmacokinetic conjectures on the particular ineffectiveness of these drugs in ED patients. In this context, the present study and the thesis that derives from it intend to focus attention also on the need for further and more in-depth studies, which will explore the pharmacokinetic and pharmacodynamic mechanisms, as well as the neurobiological reasons underlying the strong resistance of the Disorders of the Food behavior to drug treatment. More studies in this direction are necessary to improve the global and multidisciplinary management of these disorders, characterized by the highest mortality rate in the field of psychiatric diseases and which today represent a real mental health emergency, as well as a problem of public health(EpiCentro - Istituto Superiore di Sanità).
Author Disclosure
This study did not have funding sources
Author Disclosure
Contributors
Panariello F. Analized the data, managed the literature searches
and analyses and wrote the first
draft of the manuscript
Mastellari T., Wrote the protocol and collected the data
di Gianni A. Wrote the protocol and collected the data
Speciani M. Managed the literature searches
Protti M. Processed, analysed the samples and undertook the
statistical analysis
Mercolini L. Processed, analysed the samples and undertook
the statistical analysis
De Ronchi D. Designed the study and wrote the first draft of the
manuscript
Atti AR Designed the study, wrote the first draft of the
manuscript and undertook the statistical
analysis
All authors contributed to and have approved the final
manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition.
- NICE guideline (2017) Eating disorders: recognition and treatment.
- McElroy S L, Guerdjikova A I, Mori N, Keck P E (2015) Psychopharmacologic Treatment of Eating Disorders: Emerging Findings. Current Psychiatry Reports 17(5): 35.
- Claudino A M, Hay P, Lima M S, Bacaltchuk J, Schmidt U, et al. (2006) Antidepressants for anorexia nervosa. Cochrane Database Syst Rev.
- Davis H, Attia E (2017) Pharmacotherapy of eating disorders. Current Opinion in Psychiatry 30(6): 452-457.
- Himmerich H, Treasure J (2018) Psychopharmacological advances in eating disorders. Expert Rev Clin Pharmacol 11(1): 95-108.
- Jackson C W, Cates M, Lorenz R (2010) Pharmacotherapy of Eating Disorders. Nutrition in Clinical Practice 25(2): 143-159.
- Marvanova M, Gramith K (2018) Role of antidepressants in the treatment of adults with anorexia nervosa. Ment Health Clin 8(3): 127-137.
- Alañón Pardo, Ferrit Martín M, Calleja Hernández, Morillas Márquez F (2017) Adherence of psychopharmacological prescriptions to clinical practice guidelines in patients with eating behavior disorders. Eur J Clin Pharmacol 73(10): 1305-1313.
- Garner D M, Anderson M L, Keiper C D, Whynott R, Parker L (2016) Psychotropic medications in adult and adolescent eating disorders: clinical practice versus evidence-based recommendations. Eat Weight Disord 21(3): 395-402.
- Mizusaki K, Gih D, La Rosa C, Richmond R, Rienecke R D (2018) Psychotropic usage by patients presenting to an academic eating disorders program. Eat Weight Disord 23(6): 769-774.
- Kazdin A E, Fitzsimmons‐Craft E E, Wilfley D E, (2017) Addressing critical gaps in the treatment of eating disorders. International Journal of Eating Disorders 50(3): 170-189.
- Phillipou A, Rossell S L, Castle D J (2014) The neurobiology of anorexia nervosa: a systematic review. Aust N Z J Psychiatry 48(2): 128-152.
- Kaye W, Gendall K, Strober M (1998) Serotonin neuronal function and selective serotonin reuptake inhibitor treatment in anorexia and bulimia nervosa. Biol Psychiatry 44(9): 825-838.
- Barbarich N C, Mc Conaha C W, Halmi K A, Gendall K, Sunday S R, et al. (2004) Use of nutritional supplements to increase the efficacy of fluoxetine in the treatment of anorexia nervosa. International Journal of Eating Disorders 35(1): 10-15.
- Duriez P, Ramoz N, Gorwood P, Viltart O, Tolle V (2019) A Metabolic Perspective on Reward Abnormalities in Anorexia Nervosa. Trends Endocrinol Metab 30(12): 915-928.
- Paulzen M, Haen E, Stegmann B, Hiemke C, Gründer G, et al. (2016) Body mass index (BMI) but not body weight is associated with changes in the metabolism of risperidone; A pharmacokinetics-based hypothesis. Psychoneuroendocrinology 73: 9-15.
- Sigurdsson H P, Hefner G, Ben Omar N, Köstlbacher A, Wenzel Seifert K, et al. (2015) Steady-state serum concentrations of venlafaxine in patients with late-life depression. Impact of age, sex and BMI. J Neural Transm (Vienna) 122(5): 721-729.
- Uher R, Mors O, Hauser J, Rietschel M, Maier W, et al. (2009) Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord 118(1-3): 147-154.
- Unterecker S, Deckert J, Pfuhlmann B (2011) No influence of body weight on serum levels of antidepressants. Ther Drug Monit 33(6): 730-734.
- Chen G, Højer A M, Areberg J, Nomikos G (2018) Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. ClinPharmacokinet 57(6): 673-686.
- Mercolini L, Saracino M A, Protti M (2015) Current advances in biosampling for therapeutic drug monitoring of psychiatric CNS drugs. Bioanalysis 7(15): 1925-1942.
- Protti M, Mandrioli R, Mercolini L (2019) Tutorial: Volumetric absorptive microsampling (VAMS). Analytica Chimica Acta 1046: 32-47.
- Baumann P, Hiemke C, Ulrich S, Eckermann G, Gaertner I, et al. (2004) The AGNP-TDM expert group consensus guidelines: therapeutic drug monitoring in psychiatry. Pharmacopsychiatry 37(6): 243-265.
- Hiemke C, Bergemann N, Clement H, Conca A, Deckert J, et al. (2018) Consensus Guidelines for Therapeutic Drug Monitoring in Neuropsychopharmacology: Update 2017. Pharmacopsychiatry 51(1-2): 9-62.
- Mandrioli R, Mercolini L, Saracino M A, Raggi M A (2012) Selective serotonin reuptake inhibitors (SSRIs): therapeutic drug monitoring and pharmacological interactions. Curr Med Chem 19(12): 1846-1863.
- Burke M J, Preskorn S H (1999) Therapeutic drug monitoring of antidepressants: cost implications and relevance to clinical practice. Clin Pharmacokinet 37(2): 147-165.
- Lundmark J, Bengtsson F, Nordin C, Reis M, Wålinder J (2000) Therapeutic drug monitoring of selective serotonin reuptake inhibitors influences clinical dosing strategies and reduces drug costs in depressed elderly patients. Acta Psychiatr Scand 101(5): 354-359.
- Ostad Haji E, Mann K, Dragicevic A, Müller M J, Boland K, et al. (2013) Potential cost-effectiveness of therapeutic drug monitoring for depressed patients treated with citalopram. Ther Drug Monit 35(3): 396-401.
- Simmons S A, Perry P J, Rickert E D, Browne J L (1985) Cost-benefit analysis of prospective pharmacokinetic dosing of nortriptyline in depressed inpatients. J Affect Disord 8(1): 47-53.
- Bauer L (2014) Applied Clinical Pharmacokinetics 3rd ed.
- Khan A, Schwartz K A, Kolts R L, Brown W A (2007) BMI, sex, and antidepressant response. J Affect Disord 99(1-3): 101-106.
- Delahaye L, Dhont E, De Cock P, De Paepe P, Stove C P (2019) Volumetric absorptive microsampling as an alternative sampling strategy for the determination of paracetamol in blood and cerebrospinal fluid. Anal Bioanal Chem 411(1): 181-191.
- Kip A E, Kiers K C, Rosing H, Schellens J H M, Beijnen J H, et al. (2017) Volumetric absorptive microsampling (VAMS) as an alternative to conventional dried blood spots in the quantification of miltefosine in dried blood samples. J Pharm Biomed Anal 135: 160-166.
- Kok M G M, Fillet M (2018) Volumetric absorptive microsampling: Current advances and applications. J Pharm Biomed Anal 147: 288-296.
- Mercolini L, Protti M, Catapano M C, Rudge J, Sberna A E (2016) LC-MS/MS and volumetric absorptive microsampling for quantitative bioanalysis of cathinone analogues in dried urine, plasma and oral fluid samples. J Pharm Biomed Anal 123: 186-194.
- Li R R, Sheng X Y, Ma L Y, Yao H X, Cai L X, et al. (2016) Saliva and Plasma Monohydroxycarbamazepine Concentrations in Pediatric Patients With Epilepsy. Ther Drug Monit 38(8): 365-370.
- Dwivedi R, Singh M, Kaleekal T, Gupta Y K, Tripathi M (2016) Concentration of antiepileptic drugs in persons with epilepsy: a comparative study in serum and saliva. Int J Neurosci 126(11): 972-978.
- Baumann P, Tinguely D, Koeb L, Schöpf J, Le P K (1982) On the relationship between free plasma and saliva amitriptyline and nortriptyline. Int Pharmacopsychiatry 17(3): 136-146.
- Patteet L, Maudens K E, Morrens M, Sabbe B, Dom G, et al. (2016) Determination of Common Antipsychotics in Quantisal-Collected Oral Fluid by UHPLC-MS/MS: Method Validation and Applicability for Therapeutic Drug Monitoring. Ther Drug Monit 38(1): 87-97.
- Kloiber S, Ising M, Reppermund S, Horstmann S, Dose T, et al. (2007) Overweight and Obesity Affect Treatment Response in Major Depression. Biological Psychiatry 62(4): 321-326.
- Anoshkina Y, Costas Rodríguez, M Vanhaecke F (2017) Iron isotopic analysis of finger-prick and venous blood by multi-collector inductively coupled plasma-mass spectrometry after volumetric absorptive microsampling. J Anal At Spectrom 32(2): 314-321.
- De Kesel P M M, Lambert W, Stove C P (2015) Does volumetric absorptive microsampling eliminate the hematocrit bias for caffeine and paraxanthine in dried blood samples? A comparative study. Anal. Chim Acta 881: 65-73.
- Heussner K, Rauh M, Cordasic N, Menendez Castro C, Huebner H, et al. (2017) Adhesive blood microsampling systems for steroid measurement via LC-MS/MS in the rat. Steroids 120: 1-6.
- Kopp M, Rychlik M (2017) Assessing Volumetric Absorptive Microsampling Coupled with Stable Isotope Dilution Assay and Liquid Chromatography-Tandem Mass Spectrometry as Potential Diagnostic Tool for Whole Blood 5-Methyltetrahydrofolic Acid. Front Nutr 4: 9.
- Marahatta A, Megaraj V, Mc Gann P T, Ware R E, Setchell K D R (2016) Stable-Isotope Dilution HPLC-Electrospray Ionization Tandem Mass Spectrometry Method for Quantifying Hydroxyurea in Dried Blood Samples. Clin Chem 62(12): 1593-1601.
- Nys G, Gallez A, Kok M G M, Cobraiville G, Servais A C, et al. (2017) Whole blood microsampling for the quantitation of estetrol without derivatization by liquid chromatography-tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis 140: 258-265.
- Protti M, Rudge J, Sberna A E, Gerra G, Mercolini L (2017) Dried haematic microsamples and LC-MS/MS for the analysis of natural and synthetic cannabinoids. J Chromatogr B Analyt Technol Biomed Life Sci 1044-1045: 77-86.
- Ostad Haji E, Hiemke C Pfuhlmann B (2012) Therapeutic drug monitoring for antidepressant drug treatment. Curr Pharm Des 18(36): 5818-5827.
- Kotlyar M, Carson S W (1999) Effects of obesity on the cytochrome P450 enzyme system. Int J Clin Pharmacol Ther 37(1): 8-19.
- Rahmioglu N, Heaton J, Clement G, Gill R, Surdulescu G, et al. (2011) Genetic epidemiology of induced CYP3A4 activity. Pharmacogenetics and Genomics 21(10): 642-651.
- Tomankova V, Anzenbacher P, Anzenbacherova E (2017) Effects of obesity on liver cytochromes P450 in various animal models. Biomedical Papers 161(2): 144-151.
- Dwivedi R, Gupta Y K, Singh M, Joshi R, Tiwari P, et al. (2015) Correlation of saliva and serum free valproic acid concentrations in persons with epilepsy. Seizure 25: 187-190.
- General information. Epidemiology for public Health.
- Schwartz J B (2007) The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin Pharmacol Ther 82(1): 87-96.

 Research Article
Research Article