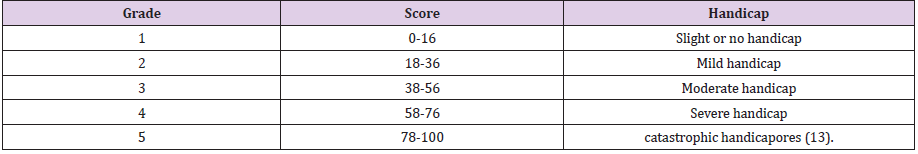
Abstract
Background: This pilot study aimed to evaluate the feasibility effectiveness and safety of an endothelial protector drug, such as Aminapthone, for patients with chronic idiopathic tinnitus.
Methods: 16 consecutive out-patients afferent to our ENT Unit from May 2017 to June 2018 and affected by chronic idiopathic tinnitus and hearing loss were enrolled in this pilot study. All the included patients presented tinnitus that worsened their Quality of Life (QoL) from more than 6 months. All the patients presented hearing loss and performed vestibular-evoked potentials and a Skull Nuclear Magnetic Resonance to avoid central causes of hearing loss/tinnitus. AMINAPTHONE (75 mg cps bid) has been administered for six months and at baseline (T0) after 3 months (T1) and after 6 months (T3) have been evaluated audiometric parameters together with a Tinnitus Handicap Inventory (TIH) scales. All the patients continued their standard of care during AMINAPTHONE treatment. The primary end point was the regression or the non-worsening of THI during the 3 – 6 months of the observational period according to the audiometric parameters, the secondary end point was the compliance to drug treatment and the drop-out of patients from the protocol study.
Results: After 3 months of treatment the medium score of THI was statistically significant decrease from 34.37 to 27.81 (p=0.027) while after 6 months (T2) THI was 30.75 with a non-statistically significant decrease between T0 and T2 (p = 0.107). Surprisingly all the patients concluded the six months treatment period confirming the good compliance to the test treatment. In addition, no adverse effects related to the treatment were recorded in the 16 patients enrolled. Conclusions: The results of this pilot observational study seem to confirm the approach with an endothelial protector drug, such as AMINAPTHONE, in the treatment of Idiopathic Tinnitus. The improvement of THI together with the good domiciliary compliance to the test treatment seems to support “the microcirculatory etiopathogenesis” of the subjective idiopathic tinnitus.
Keywords: Aminapthone; Idiopathic Tinnitus; Endothelial Protector Drug; Quality of Life; Microcirculatory Disorders; THI
Introduction
Subjective Idiopathic Tinnitus is a common and disturbing condition characterized by an auditory perception of sound or noise that has no external source [1,2]. Almost 5% to 10% of people suffer from such condition (3-5 di 1) and this rate could increase up to 30% among the elderly [3]. Tinnitus very often becomes a “chronic condition” and may result in depression, anxiety, insomnia and, especially, “poor Quality of Life (QoL) in patients with such symptom. Currently no highly effective management therapy was recommended for Tinnitus despite a lot of treatments have been tried to control this condition. Microcirculatory disorders of the inner ear with a hemodynamic and metabolic imbalance of the endothelial leading to a damage of the cochlear cells, are often bring into play in the classical cochleovestibular symptoms such as tinnitus [4]. From the literatures seems to be clear the relationship between inner ear microcirculation dearrangements and Tinnitus: this is the reason why we try to use “an endothelial protector drug” such as AMINAPTHONE™, that has recently demonstrated to be effective in down-regulates molecules with key vasoactive roles such as E-Selectine and Endothelin 1 (ET-1) expression in vitro and in vivo [5-7] and to modulate the expression of endothelial inflammatory molecules that contribute to pathogenesis of several conditions where the endothelial dysfunction may play the main role, in particular in early events [8,9].
Materials and Methods
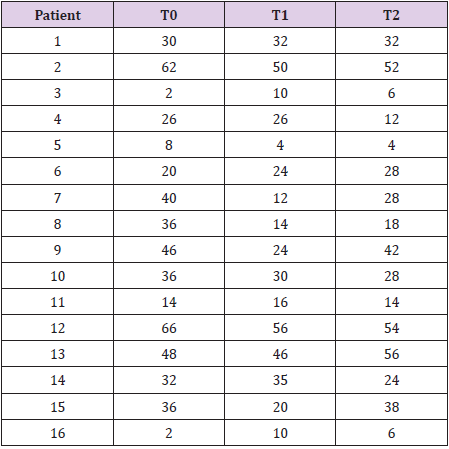
We have retrospectively analyzed data of sixteen patients (medium age 61.87 +/-13.68 years, 9 women and 7 men), affected by idiopathic chronic tinnitus afferent to ENT Unit of Ramazzini Hospital in Carpi (Modena) from May 2017 to June 2018. Table 1 shows the main characteristics of the patients. All the patients enrolled in this observational study aged more than 18 years, presented Tinnitus from minimum six months that worsened their Quality of Life (QoL) together with hearing loss. They performed vestibular evoked potentials and Skull Nuclear Magnetic Resonance to avoid central causes of hearing loss/Tinnitus. All the patients add to their standard of care AMINAPTHONE (75 mg cps bid) for 6 months and were evaluated for audiometric parameters and for their subjective perception of Tinnitus by using the Tinnitus Handicap Inventory (THI) scale [10-12] at baseline (T0) after 3 months (T1) and after 6 months (T2) of treatment (Table 2). We exclude from the analysis patients with inflammatory ear pathology or retrocochlear neurologic or neurosurgery pathologies; all patients had already tried other therapy without significant benefits. We exclude also patients taking steroid-drugs or NSAIDs (nonsteroidal antiinflammatory drugs).
Table 1: Characteristics of the population: We enrolled 16 patients, 7 male and 9 females with tinnitus. Every patient has been tested with hearing measurement, Auditory Brainstem Response and Magnetic Resonance Imaging of head with Gadolinium.
Note: *Legend: PTA: pure tone audiometry is the medium value in 250-500-1000-2000-4000Hz and is expressed in dB; Changing PTA: if hearing threshold medium levels changes during this six-months study; we consider a changing if there is a difference more than 10dB; Tinnitus site: the ear in which patient refers tinnitus; when patient refers bilateral (bil), we indicated the worst side. Characteristic of tinnitus: we indicated characterizes of tinnitus in frequency (Hz) and intensity (dB).
Considering the hearing loss of all the patients and the
chronicity of their pathologies an improvement in their QoL relate
to subjective TINNITUS measured by THI scale at 3rd or 6th months
was considered as primary end point. Secondary end-points was
considered the “compliance of the patients” to the study drug
together with the number of “drop-out patients” during the 6th
months of treatment. A study of the “intra-patient” THI values,
according to the audiometric parameters, was also performed in
order to detect the correlation between a possible worsening of the
audiometric parameters due to the natural course of the chronical
pathology and THI values improvement/impairments.
The outcome values were conducted by using SAS package
8.1 (SAS Institute Inc, Cary, NC, USA): differences in demographic
and clinical variable were evaluated by using T-Test in the cae
of continuous variables. A p value of <0.05 was considered as
statistically significant. All patients gave informed, signed consent
to participate in this study.
Results
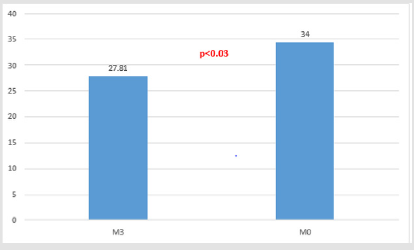
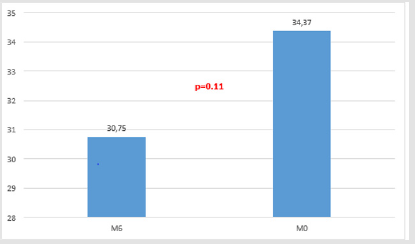
All the 16 patients enrolled in this observational retrospective study complete the 6-month study period. No one patients presented adverse effects related to the study drug during the same time. Table 3 shown the results for single patient related to the TINNITUS- Quality of Life (THI). THI mean value at T0 (baseline) was 34.37 +/-17.83 while the same value significantly decreases to 27.81+/-15.44) (p<0.03) after three months of treatment (Figure 1). After 6 months of treatment (T2) THI mean value was 30.75 +/- 17.58 with a non-statistically significant p-value decrease (p=0.11) versus T0 (THI mean value = 34.37 +/- 17.83) (Figure 2).
Referring to the secondary end-points (drop-out of the patients from the study during the sixth months of treatment and patient’s compliance to treatment) all patients follow the six months courses of the Aminapthone treatment without adverse effects and with a good compliance to treatment: considering the kind of patients who always research a resolutive and immediately tinnitus treatment after have tried many other pharmaceutical an non treatment and the “usually loosing patients during a long- time treatment” we can conclude that Aminapthone treatment was “well-tolerated” (or well-accepted) from patients with a “good personal satisfactory index” for Aminapthone treatment and that, consequently, we have reach the secondary end-point of the study [13].
Discussion
Tinnitus is one of the most common hearing disorders; many
risk factors has been individualized for this “stressing condition”
such as age, hearing loss, inflammatory diseases or tumors of
the ear, head or vertebral cervical trauma, ototoxic drugs, noise
exposure and psychological disorders. Tinnitus could be a life-long
disorder bringing to anxiety, depression, insomnia, hyperacusis,
concentration difficulties and, sometimes, to suicide. QoL of people
affecting by Tinnitus is often worsened leading to transform this
hearing disorder in a “very serious disorder” involving “all the personal activity’s area” of the patient. The inner ear diseases are
often caused by microcirculatory disorders [14].
Figuerado and Coll, reviewing the association between arterial
hypertension and Tinnitus conclude that “there is evidence of an
association between Tinnitus and Hypertension “and that “changes
in the cochlear microcirculation, resulting in hearing loss, may be
an adjuvant factor in Tinnitus pathophysiology” [15]. Evidence of
literatures suggest that restore hemodynamic and dysmetabolic
imbalance responsible for cochleovestibular microcirculatory
disorders could represent a therapeutic strategy to control
primary idiopathic Tinnitus. Considering that phenomenon
such as secretions of endothelial pro-inflammatory molecules,
hyperexpression of Vascular Cells Adhesion Molecules (VCAM)
and Endothelial Leukocytes Adhesion Molecules (ELAM) and
hyperexpression of Endothelin 1 (ET-1), seems to be “the key
player” in the microcirculatory disorders.
The use of an endothelial protector drug such as
AMINAPTHONE™ [16] could represent a new and interesting
pharmacological approach in the “multidisciplinary treatment”
of Primary Idiopathic Tinnitus. The results of this retrospective,
observational study performed on 16 outpatients affected by
Chronic Idiopathic Tinnitus seems to confirm this “endothelial
protector approach”. QoL of Tinnitus patients measured by THI
significantly improves (p = 0.03) immediately after 3 months
of treatment and this improvement was confirmed, also if a
non-statistically significant manner, after 6 months (p = 0.11).
Interestingly, in the only 6 patients in which there were a
deterioration of the THI at 6 months, we have observed a worsened
in the audiometric performance: probably “the worsening of
idiopathic chronic hearing loss pathology” was too strong to allow
to endothelial protection to control Tinnitus. Nevertheless, the
primary end-point of this observational study (THI improvement)
has been significantly achieved after 3 months of treatment with
AMINAPTHONE; probably an “plateau- effect” do not permit to
obtain a statistically significative improvement after 6 months of
treatment, but in all cases, there were no regression-effect.
At the same time the study secondary end-point has been
completely achieved because no one patient stop to keep the
study drug and the compliance of the patient to the six months
study treatment was good in the 100% of the patients. This was
a very interesting observation especial if confronted with our
previous experience: the use of a new drug in patient affected by
Tinnitus was immediately stopped in a lot of patients because
of the “stress conditions” of the same patients. Obviously this
study has a lot of methodological limitations such as the design
of the study, the little number of patients enrolled in the trial, the
retrospective- observational methodology, together with the open
and retrospective protocol design so that every observational
results must be interpreted with caution. In future a prospective,
controlled, double blind clinic study may confirm these preliminary
and pivotal results.
In conclusion, there is an association between TINNITUS and
microcirculatory disorders. This association cannot be dissociated
from the hearing loss, which was also more prevalent among
Tinnitus patients. The use of drugs able to protect the endothelium
damages has not been very well tested. Aminapthone is the first
drug of a new drug’s category: “the endothelial-protector drugs”.
The preliminary and pilot results of this observational study seem
to encourage the use of this new pharmacological treatment in
terms of Qol improvement of patients and of patients’ compliance to
treatment. In future a prospective, controlled, randomized, double
blind clinic study may confirm these preliminary but interesting
results.
References
- Tamir SO, Marom T, Shushan S (2018) Tinnitus Perspectives among Israeli Ear, Nose and Throat Physicians: A Nationwide Survey. J Int Adv Otol 14(3): 437-442.
- Bhatt JM, Lin HW, Bhattacharyya N (2016) Prevalence, Severity, Exposures, and Treatment Patterns of Tinnitus in the United States. JAMA Otolaryngol Head Neck Surg 142(10): 959-965.
- Lasisi AO, Abiona T, Gureje O (2010) Tinnitus in the elderly: Profile, correlates, and impact in the Nigerian Study of Ageing. Otolaryngol Head Neck Surg 143(4): 510-515.
- Neri S, Signorelli S, Pulvirenti D, Agostino Serra, Alkaterini Tsami, et al. (2006) Oxidative stress, nitric oxide, endothelial dysfunction and tinnitus. Free Radic Res 40(6): 615-618.
- Lenna S (2006) Novel mode of action of the aminaphtone: down-regulation of a E-Selectine expression in ECV- 304 cells. Int Angiology 25: 189.
- Scorza R, Santaniello A, Salazar G, Karen Toussoun, Lorenzo Beretta, et al. (2008) Effects of aminaftone 75 mg TID on soluble adhesionmolecules: a 12- week, randomized, open-label pilot study in patients with systemic sclerosis. Clin Ther 30(5): 924-929.
- Scorza R, Santaniello A, Salazar G (2008) Aminaftone, a derivative of 4-aminobenzoic acid, downregulates endothelin-1 production in ECV304 Cells: an in vitro Drugs R D 9(4): 251-257.
- Salazar G, Bellocchi C, Todoerti K (2016) Gene expression profiling reveals novel protective effects of Aminaphtone on ECV304 endothelial cells. Eur J Pharmacol 782: 59-69.
- Salazar G, Bellocchi C, Todoerti K, Luca Piacentini, Raffaella Scorza, et al. (2016) Time-course gene expression data on the transcriptional effects of Aminaphtone on ECV304 endothelial cells. Data Brief 8: 836-8350.
- Newman CW, Jacobson GP, Spitzer JB (1996) Development of the Tinnitus Handicap Inventory. Arch Otolaryngol Head Neck Surg 122(2): 143-148.
- Moschen R, Fioretti A, Eibenstein A, Eleonora Natalini, Domenico Cuda, et al. (2018) Validation of the Italian Tinnitus Questionnaire Short Form (TQ 12- I) as a Brief Test for the Assessment of Tinnitus-Related Distress: Results of a Cross-Sectional Multicenter- Study. Front Psychol 9: 65.
- Monzani D, Genovese E, Marrara A (2008) Validity of the Italian adaptation of the Tinnitus Handicap Inventory; focus on quality of life and psychological distress in tinnitus-sufferers. Acta Otorhinolaryngol Ital 28(3): 126-134.
- Neri G (2018) Sulodexide nell’acufene soggettivo idiopatico: Update 3009-2018. OTO Neurologia 49: 17-21.
- Neri G, Marcelli V, Califano L (2018) Glicover Investigators. Assessment of the effect of mesoglycan in the treatment of audiovestibular disorders of vascular origin. Int J Immunopathol Pharmacol.
- Figueiredo RR, de Azevedo AA, Penido Nde O (2015) Tinnitus and arterial hypertension: a systematic review. Eur Arch Otorhinolaryngol 272(11): 3089-3094.
- Bertini M, Battista Gervasi G, Baldacci M (2017) Aminapthone: a drug for microcirculation de-arrangement: novel aspects of an endothelial protector drug. ESM-EVBO 2017. 2nd Joint Meeting of the European Society for Microcirculation (ESM) and European Vascular Biology Organisation (EVBO). Geneva, Switzerland, 29 May to 1 June 2017: Abstracts. J Vasc Res:44: 6C-04.

 Research Article
Research Article