Abstract
Background: Endometriosis is a hormone-dependent benign inflammatory disease of the female reproductive system characterized by the presence of endometrium in an abnormal or ectopic location causing pain & infertility and affects approximately 10% of reproductive-age women. A more in-depth insight into the complex mechanisms involved in the commencement, development, and advancement is required to accomplish the desired objectives of therapy effective against the symptomatic disease, associated infertility, and malignancies.
Methods: We searched the PubMed database using the following search terms in various combinations “Endometriosis and Pathogenesis, Infertility, Pregnancy, Cancer, Biomarkers, Risk factors, Management” and identify relevant English language studies published up to June of 2020. Furthermore, articles were included within the citing references.
Results: This review has been structured from a historical perspective that facilitates an up-to-date comprehension of the disease, illustrated by relatively recent molecular and genetic signs of progress.
Conclusion: This article will furnish the readers with up-to-date knowledge regarding the biomarkers, infertility, pregnancy, and carcinoma with the ailment and also shed light on the management of the disease.
Keywords: Cancer; Endometriosis; Infertility; Management; Pathogenesis
Abbreviations: ASRM: American Society for Reproductive Medicine; EFI: Endometriosis Fertility Index; QoL: Quality of Life; MRI: Magnetic Resolution Image; TVUS: Transvaginal Ultrasonography; DIE: Deep Infiltrating Endometriosis; CT: Computerized Tomography; MDCT-e: Multidetector CT Enema; MRI-e: MRI Enema; BMI: Body Mass Index; EAOC: Endometriosis-Associated Ovarian Cancer; RTKs: Receptor Tyrosine Kinase; ART: Assisted Reproductive Technology; NSAIDs: Non-Steroidal Anti-Inflammatory Drugs; COX: Cyclooxygenase; ASA: Acetylsalicylic Acid; GnRH: Gonadotropin-Releasing Hormone; ADR: Adverse Drug Reaction; OCP: Oral Contraceptive Pill; COC: Combined Oral Contraceptives; CI: Confidence Interval; FDA: Food Drug Administration; CHC: Combined Hormonal Contraceptive; Ais: Aromatase Inhibitor; VEGF: Vascular Endothelial Growth Factor; CPI: Cell Proliferation Index
Introduction
Endometriosis is defined as a hormone-dependent benign inflammatory disease of the female reproductive system characterized by the implantation and growth of lesions formed by endometrial stromal and epithelial cells present outside the uterine cavity, mainly in the peritoneum associated with abdominal viscera [1-3]. However, it is stated by some studies that glandular and stromal cells can be present outside the uterus such as on the surfaces of ovaries and the pelvic [4]. Various kinds of literature have suggested that endometriotic lesions are not restricted within the boundaries of the abdominopelvic cavity. Still, it is also found in other parts of the body, such as gastrointestinal tract, lungs, diaphragm, abdomen, and pericardium [5,6]. The urinary tract, the thorax, and the nasal mucosa [7], liver [8]. Also, endometriotic foci have been found on the uterine ligaments, cervix, labia, and vagina [9] peripheral and axial skeleton, as well as the central nervous system [10]. Although it is benign, it happens spontaneously in women and non-human primates that menstruate, causing pain and infertility [11].
Prevalence
Endometriosis affects approximately 6% to 10% that covers 176 million women of childbearing age, as high as 35-50% women with pain or unexplained infertility [12] and becoming a substantial socioeconomic burden globally [5,6]. However, an actual incidence of endometriosis is quite difficult to rule out due to expensive diagnosis and surgical based procedures, so the estimated range varies from 10%-15% [13]. The general assessment indicates about 25% of all women may have endometriosis in their thirties to forties and after diagnoses for hospitalizations for genito-urinary issues result in 6.9% disease involvement [14]. The pelvic region comprises three significant forms of endometriosis such as ovarian, peritoneal, and infiltrating endometriotic lesions. Morphologically, lesions are divided into three groups viz; white, red, and black lesions. The red lesion interprets with a high level of vascularization, later followed by whitish lesion due to inflammation and fibrosis and classical black lesions are characterized by cyclic tissue decomposition and healing with the subsequent formation of scar tissue [6].
Classification (The Revised ASRM Criteria for Endometriosis Staging)
The American Fertility Society score which is presently better known as American Society for Reproductive Medicine (ASRM) named based on data obtained from the operating room and comparative study of therapeutic interventions which is widely accepted classification among clinicians [15]. ASRM classifies the severeness of endometriosis based on location, number, morphology, and size of the lesions found during the surgical procedures into point score from Stage I (minimal) to Stage IV (severe) [16] (Table 1). The ASRM classification system’s major limitation remained unclear for many years, whether it has any prognostic significance regarding the prediction of a woman’s fertility potential after surgery, which is paramount for patients trying to conceive. Endometriosis Fertility Index (EFI) is being more recent classification because it includes point scores from the ASRM system combined with additional anamnestic, and postsurgical information [17]. EFI is a simple, robust, and validated clinical tool employed for setting the management plan of endometriotic infertile women that predicts pregnancy rate after the surgical staging of endometriosis. It furnishes reassurance to those women with good predictions and avoids wastage of both time and treatment for those with poor prognoses [18].
Table 1: The staging system of endometriosis.
Note: This table explains about the staging system of endometriosis. ASRM classifies the severeness of endometriosis based on location, number, morphology, and size of the lesions found during the surgical procedures into point score from Stage I (minimal) to Stage IV (severe).
Clinical Features
Despite being a common gynecological disease, endometriosis can be either asymptomatic or symptomatic, and most commonly it is characterized by cyclic menstrual pain, chronic pelvic pain, dyspareunia, menorrhagia, and dyschezia [19] as well as dysuria, back pain and bladder or bowel problems (for instance, painful urination or bowel movements) [20]. The pelvic pain in women with endometriosis is described as pain before the onset of menses and deep dyspareunia that worsens upon menstrual flow. Sacral and lower backaches during menses also can be present [21].
Societal and Financial Impacts of Endometriosis
Endometriosis has a great impinge not only physically but
also on mental health, social life, and daily activities of living,
productivity and ultimately become a significant economic and
social burden on patients, their families & relatives, and society as
a whole. Notwithstanding its increasing prevalence and expensive
diagnosis & management, the present understanding of the disease
is limited due to lack of fund and advanced research causes a
slowdown of the needed innovation in the field of diagnosis and
treatment [22]. The prospective study assesses the estimated
running cost of endometriosis in 10 countries stated $12 419 per
woman (approximately €9579) annually, comprising one-third of
the direct health care costs with two-thirds attributed to a loss of
productivity. This total spending seems to be a significant factor for
decreased quality of life (QoL).
This study showed that the gradual expensive diagnosis
economic burden associated with endometriosis espied in
the referral centers is high and is similar to other chronic
diseases (diabetes, Crohn’s disease, Rheumatoid arthritis) [23].
Endometriosis is estimated to have the societal burden of more
than $49 billion in the USA alone with a decline in productivity per women accounting twice high as expenses of healthcare [24]. In
defiance of advancements in the diagnosis and understanding of
endometriosis, many women with endometriosis often associated
with multiple side effects, and high recurrence rates are struggling
due to inefficient non-invasive diagnostic methods and treatments
[25,26]. The QoL of the patients is broadly affected with a negative
impact on social and family life, and high health care costs due to
endometriosis [27,28].
Risk Factors
There are several existing evidence of risk factors that may
play a role in the etiopathogenesis of endometriosis including age,
genetic factors, menstrual parameters, anthropometric measures,
body habitus, lifestyle factors, and environmental exposures.
Endometriosis is one of the salient diseases seen in the reproductive
age of women which may be explained by estrogenic atmosphere
strongly implicated in its pathogenesis and considered an average
age of diagnosis being 28 years [29]. The risk of endometriosis
has been linked to ethnicity, and several studies have reported
a nine-fold increase in Asian women’s risk when compared to
the European-American white female population. A manifold of
education has been said the link with ethnicity as Asian women
have nine times greater risk than the European- American white
female [30]. Nonetheless, black women appear less likely to be
diagnosed with endometriosis compared with white women [31].
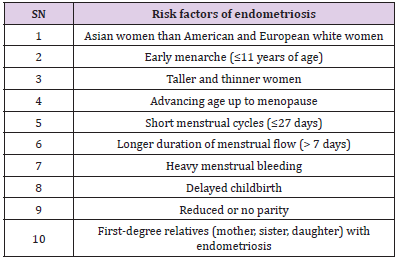
Some reproductive problems such as early menarche (≤11 years
of age), short menstrual cycles (≤27 days), heavy and long-lasting
bleeding, reduced parity and reduced lifetime duration of lactation
collaborate with the increased risk of endometriosis [2,15,32].
Endometriosis is inversely correlated with body weight, body mass
index, and waist-to-hip ratio. Besides, it is more common in taller
and thinner women due to consistently elevated levels of estradiol
in follicular phase [33]. The altered endangerment of endometriosis
has been reported in association with certain lifestyle factors such
as smoking, exercise, and consumption of alcohol and caffeine [34].
The risk of endometriosis appears to be increased in women who
are associated with the following pictures [2,30,35,36] (Table 2).
Table 2: Risk factors in association with endometriosis.
Note: Table 2 explains the relationship between endometriosis and several risk factors. The risk of endometriosis has been linked to ethnicity, race, age, body mass index, height and other diseases.
Role of Animal Model in Diagnosis
The conventional discovery and development of diagnostic markers & new therapeutic drugs have been handicapped due to the unavailability of suitable animal models for in vitro testing and their limited use [37]. Thus, the researcher believes that an appropriate model’s evolution may occupy a strong position to reduce the prevalence rate and provide quality life to many females of the younger generation. They stated that humans and non-human primates are the only mammal option that spontaneously develops endometriosis. Still, the limitations of humans and expenses of primate studies have made a boundary to the researcher to make use of small animal models. Non-primates could be another possible option for medical research. However, unfortunately, they neither menstruate nor are capable of developing endometriosis. Even so, several researchers decided to use rabbits, rats, and mice to investigate multiple aspects of the disease. Although several studies have proven valuable for studying some aspects of the disease, surgical induction of endometriosis lacks the potential to address the underlying cellular mechanisms of endometrial attachment and invasion that are critical for the initial establishment of ectopic lesions in women.
Therefore, researchers moved forward to establish the best animal model with similar pathophysiology of human disease [38]. Among various animal models of endometriosis, rodent models have a plethora of benefits such as; cheaper due to their small size, large litters, and short gestation. Additionally, these animal models play a significant role in the study of endometriosis because of the wide availability of genetic knock-out mice, antibodies against murine and rat proteins as well as knowledge of the murine genome. Many different model systems have been generated in mice and rats, each with specific characteristics that are consequential for the interest of study [39]. Females with very early stages of the endometriosis are rarely diagnosed, making it difficult to scrutinize the elaboration and progression of ectopic lesions in a large population. Numerous experimental models of endometriosis have been developed to fulfil these crucial gaps, which can explain the pathophysiology and the consequences of this [38]. More significantly, rodent models of endometriosis are often utilized for preclinical testing to identify new therapeutic agents. The usage of experimental endometriosis models for therapeutic testing has recently been reviewed.
Pathogenesis
Even after decades of research the exact cause of endometriosis is not yet fully comprehended. However, existing documents suggest that combinations of hormonal, immunologic, genetic, and environmental factors are contributing to the origin and development of endometriosis [3]. There are several leading mechanisms like retrograde menstruation, altered immunity, coelomic metaplasia, and metastatic spread. Moreover, the other possible pathogenic elements like hormonal imbalance, immunological, environmental toxin, genetic, and epigenetic mechanisms may occur to allow the disease establishment and progression [40]. Eventually, stem cell and genetic emergence of the disease are also being suggested for undergoing novel research. In 1940, Sampson proposed a distinctive theory in connection with the pathogenesis of endometriosis called retrograde menstruation, which is a widely accepted theory. Normally, endometrium sheds sloughs off through the cervix and vagina during the menstrual phase of the cycle, but sometimes it moves upward and backward through the fallopian tube into the peritoneal cavity known as retrograde menstruation.
was found that approximately 90% of the subjects were estimated to experience a certain amount of endometrial sheds into their peritoneal cavity. Still, only a small proportion of them have developed endometriosis [41]. However, some authors explained that retrograde menstruation in association with other abnormalities like eutopic anomalies of the endometrium (such as; the altered synthesis and secretion of proteins and gene expression) and immune system abnormalities might be involved in the pathophysiology of endometriosis [42]. Whereas other researchers have hypothesized that pathogenesis of endometriosis for the development of ectopic lesions is enhanced by a replicate capacity due to a surge in telomerase synthesis in the sloughed endometrium during retrograde [43]. Meanwhile, induction theory was hypothesized by another researcher which includes: the sloughing endometrium gives off “factors” during menstruation, and that causes changes in the surface epithelium of the ovary leading the differentiation of endometrium-like tissue and migration to the peritoneal cavity.
According to in situ theory, the totipotent cells endure homogenous from the fetus surviving into adultness and during the reproductive age, get stimulated and thus differentiating into endometriotic lesions [44]. There might be a certain contribution of endometrial stem cells in the etiology of the disease which describes certain rare and extreme cases of endometriosis. Pathogenesis of endometriosis most likely involved various factors, and extensive investigation has explored the role of genetics, environmental factors, and the immune system in predisposing patients. A new insight into normal physiology of uterus and pathogenesis of endometriosis is found to be sought by the identification of endometrial as well as extra uterine stem/progenitor cells that aim the uterus and endometriotic implants [45].
Epigenetics of Endometriosis
Epigenetics describes changes to the cytosine base pairs and histones remodeling that affect gene expression, however, they are not mutations of the DNA itself. Several mechanisms might change the epigenetics of endometriosis; they are heritable changes in gene expression that occur without alterations to the underlying DNA sequencing [46]. As they are stable and heritable, they facilitate the propagation of the transformed cell line and can be preserved in cell divisions. Besides, they are reversible due to which, remodeling to any future cells in that line could alter the epigenetic profile further or remove it together [47]. The refashioning includes DNA methylation, histone methylation, acetylation, sumoylation, ubiquitylation, and phosphorylation, as well as chromatin remodeling and RNA transcriptional alterations [46]. There are specific genes that are known to be overexpressed in decidualization, having been seen down-regulated in endometriosis and vice versa. These comprise various elements like transcription factors, hormones, growth factors, cytokine/chemokine’s signaling, cell cycle regulation, adhesion, the immune system, and proteases, including genes associated with steroidogenesis, implantation, and placental development could also be affected [48].
Endometriosis in Association with Fertility
Fertility is affected by many pathways in the case of endometriosis such as peritoneal inflammation and endocrine imbalance, which interfere with ovarian function and ultimately reduce oocyte’s competence, but minimal and mild endometriosis has been shown to improve fertility modestly after removal of superficial foci. The chance of fertility after resection of endometriomas and deep infiltrating lesions has remained undocumented [17]. As inferred by women’s accounts, it may be salutary for drawing out relevant clinical guidelines for the clarification of evidence depicting association among endometriosis and Fertility and treatment of conditions and dissipating the shared beliefs, along with the notion that pregnancy is an appropriate alternative for a cure. Besides, it may be employed for developing the protocols for understanding and addressing the fertility concerns specific to women those who do not reveal themselves as heterosexual [49].
Endometriosis in Association with Infertility
The routine intricacy that is seen in women with moderate to severe endometriosis is infertility [5]. A large cohort study revealed an increased risk of subsequent infertility by 2-fold in women age of fewer than 35 years with a prior history of laparoscopically confirmed endometriosis [15]. During endometriosis, the inflammatory environment of Pelvic anatomy becomes distorted and results in the reduced monthly fecundity rate via mechanical interruption like pelvic adhesions. These disruption imbalances the process of oocyte release or pickup alters sperm motility, interferes with the myometrium contraction ability, impairs fertilization, and embryo transport. Also, gamete transport and embryo implantation are affected due to escalation in cytokine production, which reduces the tubal function and decline in the tubal motility and contractility. The current evidence proposed the mechanisms of endometriosisassociated with infertility [50].
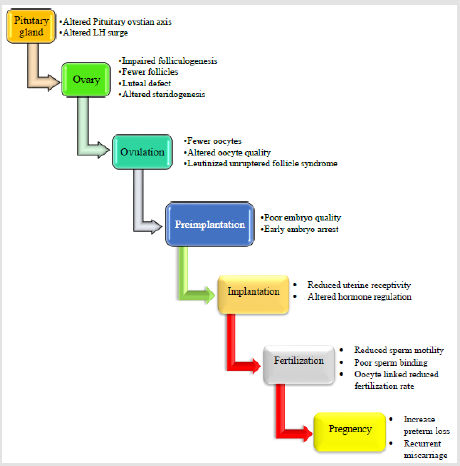
Impairment of the hypothalamo-pituitary-ovarian axis may kick into infertility in women carrying with a prolonged follicular phase, decreased serum estradiol levels, and reduced peak luteinizing hormone concentration [51]. The principal reason for morbidness in women with endometriosis is infertility. In people who have endometriosis, 30 to 50 percent face infertility, and consequently, fecundity decreases from 15 to 20 percent each month in healthy women up to 2 to 5 percent in those with endometriosis [34,52,53]. According to history, women having endometriosis-associated infertility are prone to minute, exact or multidimensional abnormalities [51,54-57] (Figure 1).
Figure 1: Endometriosis in association with infertility.
Note: Endometriosis has been associated with infertility. However, this figure tries to explain the mechanisms by which endometriosis affects gametes and embryos, the fallopian tubes and embryo transport, implantation and the eutopic endometrium; these abnormalities begins from altered pituitary ovarian axis leading to infertility.
Endometriosis in Association with Sub-Fertility
Endometriotic lesions may vary from just a few implants to extensive adhesions and organ infiltration and even outside the pelvic cavity, validated by ASRM classification into four classes: minimal, mild, moderate, and severe endometriosis [58]. There were several pieces of evidence to support the hypothesis that the presence of endometriosis causes subfertility. This evidence includes an increased prevalence of endometriosis in sub fertile women compared with women of proven Fertility, a reduced monthly fecundity rate (MFR) in baboons with mild to severe endometriosis in comparison to those with minimal endometriosis or a normal pelvis, a trend toward a reduced MFR in infertile women with minimal to mild endometriosis when compared to women with unexplained infertility, a reduced MFR and cumulative pregnancy rate after donor sperm insemination in women with minimalmild endometriosis compared with those with a normal pelvis, a reduced implantation rate per embryo after IVF in women with moderate to severe endometriosis in comparison to women with a normal pelvis, and an increased MFR and cumulative pregnancy rate after surgical removal of minimal to mild endometriosis [59].
Endometriosis and Pregnancy
Endometriosis is a disease of women’s reproductive period. The individual with a previous diagnosis of the disease had adverse consequences such as an increased risk of pregnancy and neonatal complications, and this risk remained significant after adjusting for maternal age. The risk of multiple pregnancies, breech presentation, and placenta previa was more than two-fold higher in women with endometriosis than in the control group. Besides, the estimated risk of stillbirth was more than 1.5-fold higher in the affected group. Endometriosis does not influence fetal well-being because the pregnant mothers with endometriosis are at higher risk of obstetric complications like miscarriages, threatened miscarriages, preterm labor, preterm birth, and a higher cesarean section rate [60]. Women with endometriosis are more vulnerable to serious and important adverse maternal, fetal and neonatal outcomes [61]. As revealed by a large study, people with endometriosis had greater odds of pre-eclampsia (OR 1.13, 95% CI 1.02-1.26), antepartum bleeding/placental complications (OR 1.76, 95% CI 1.56-1.99), and cesarean delivery (OR 1.47, 95% CI 1.40-1.54) [62].
Diagnosis
Primarily, those suspected patients are screened by physical
examinations and pelvic ultrasound. Secondarily, further
investigations are performed such as target pelvic examination,
transvaginal ultrasound, and pelvic magnetic resolution image
(MRI) by an expert [63]. The gold standard of diagnosing the
endometriosis clinically is a histopathological examination that is
many times undergone by a laparoscopy. If a woman has a thorough
history of following clinical features such as; recurring or persistent
pelvic pain, dysmenorrhoea, dyspareunia, and infertility along with
physical symptoms like; pain during pelvic examinations, nodules
in the uterosacral ligament, retrocervix and vaginal fornix, as well
as vagina and rectum is advised for a diagnostic laparoscopy to
confirm the endometriosis [64].
Imaging technology occupies a significant position in the
diagnosis of the disease. However, in the case of endometriosis,
it is imperative not only for assessing the extent of disease; but
also sets a guideline for surgical excision of the targeted area. The advancements in radiological techniques namely; transvaginal
ultrasonography (TVUS) or Magnetic Resonance Imaging (MRI)
are very useful clinically in symptomatic patients [65]. Pelvic
endometriosis comprises three major separate commodities such
as peritoneal, ovarian, and deep infiltrating endometriosis (DIE)
with different pathogenesis [66]. Also, the multislice computerized
tomography (CT) enteroclysis and multidetector CT enema
(MDCT-e) and MRI enema (MRI-e) are useful in the evaluation of
bowel endometriosis and their sensitivity and specificity are 98.7%
and 100%, respectively [67,68].
Biomarkers of Endometriosis
Researchers are exploring the utility of biomarkers for early diagnosis as a noninvasive approach, but more investment in this area is required for it to be fruitful. Current blood-based biomarkers under investigation include; regulators of gene expression (micro- RNAs), inflammatory markers, tumor markers, growth factors, and hormonal markers, proteomics, metabolomics, oxidative stress, autoantibodies as well as endometrial and menstrual effluent biomarkers. Despite extensive research in the field of biomarkers for endometriosis including blood, urine, body fluids and endometrial biopsy, neither a single biomarker nor a panel of biomarkers has been proven for a noninvasive diagnostic test which can provide sufficient sensitivity and specificity [69]. The invention of new and validated putative biomarkers are crucial for the advancement of the field and are top research priorities for endometriosis proposed in 2009 and 2013 by highly ranked researchers [23,70].
Endometriosis- Associated Cancer
The association between endometriosis and gynecological
cancer on a molecular basis is obscure. Formation of endometriallike
tissue and lesion on the surface of the uterus and fallopian
tubes cause pelvic inflammation, severe pain, and infertility [71,72].
The relationship between endometriosis and ovarian cancer was
first published in 1927 [73]. No epidemiological evidence was
published until the end of the 20th century. After that, the first
large epidemiological study popped in from Sweden along with
two big studies (20,686 and 64,492 participants respectively).
The studies revealed that there was an increased risk of ovarian
cancer for those women who were suffering from endometriosis
[74,75]. Also, there are many pathological factors involved to cause
endometriosis, which includes; early menarche, abnormal uterine
bleeding, abnormal estrogen level, low body mass index (BMI), cell
adhesion factors, angiogenic factors, elevated gonadotropins, and
chronic inflammation. According to the previous pieces of evidence
of endometriosis, the parents, siblings, children as well as twins of
those who have the disease, the likelihood of cancer may be up to
ten folds higher than the healthy population.
Moreover, single nucleotide polymorphism may increase the
risk of endometriosis and its associated cancer [76]. The overall
risk of Endometriosis-associated carcinoma remains low despite it is a common medical issue. According to a huge epidemiological
study, the all-inclusive prevalence of ovarian cancer in a patient
diagnosed with endometriosis was 0.3-0.8 percent, which was 2-3
times perilous than the controls [77]. Endometriosis is somatically
taught genetic change similar to those found in cancer, leading to
clonal expansion and genetically abnormal cells. Endometriotic
cysts are monoclonal characterized by loss of hetrozygocity
in 75 percent of them, and mostly affecting 9p, 11q, and 22q
chromosomes [78]. Jiang, X et al. investigated the possibility of
endometriosis - associated ovarian cancer. In this study, 40 cases
of endometriosis were examined for clonal status, the functional
alteration in TP53, RASK and, all losses is the candidate of ovarian
tumor suppressor on chromosome arm 6q, 9p, 11q, 17p, 17q and
22q genes were investigated [79]. The permanent and irreversible
changes of a nucleotide sequence in endometriotic lesions called a
somatic mutation, and many genetic mutations have been identified
in endometriosis-associated ovarian cancer (EAOC).
39 genes were identified in the association between
endometriosis and cancer. Several studies focused on the loss of
heterozygocity of 10q gene, chromosomal aneuploidy, genomic
alteration PGR and, ESR1 gene were common in women cancer
[71,80,81]. Sato, et al. investigated that the functional alteration
of tumor suppressor gene (PTEN/MMAC) which is located on
chromosome arm 10q, in this study 54 cases of endometrioid
carcinoma and endometrial cyst were included. The result was
suggested that LOH at this site occurs 8 of 19 endometriosis -
associated ovarian carcinomas (42.1%), 6 of 22 clear cell carcinoma
(27.3%), and 13 of 23 endometriosis cyst (56.5%). Similarly, 4 out
of 20 endometriosis - associated carcinomas having a somatic
mutation in PTEN were identified [82]. Specific protein markers for
ovarian carcinoma have been evaluated in atypical endometriosis.
Hepatocyte nuclear factor - 1β (HNF-1β) is a transcription factor
that has significantly been expressed in ovarian cancer and seldom
expressed in non-clear cell carcinoma [83]. The data collected
antecedent research suggested that endometriosis is a monoclonal
neoplastic disease and also a precursor of EAOC notwithstanding
menopause. On one hand various types of molecular events like
alteration of p53 gene, PTEN silencing, K-ras mutations have
been discovered in EAOC. While activation of HNF-1 on the other
hand, appears to be distinctive to clear cell carcinoma in becoming
apparent in Endometriosis [84].
According to contemporary studies of whole - genome or
targeted sequencing ARIDIA and PIK3CA genes were identified
as having frequent mutations while PPP2RIA and KRAS having
moderate mutations in ovarian cell carcinomas [85-87], mutations
of PTEN, CTNNB1, and KRAS in endometrioid carcinoma [83,88,89].
Activation of oncogenic KRAS and PI3K survival pathways and
inactivation of tumor suppressor genes PTEN and ARID1A in
combination with the results of gene expression profiling of these
two tumor types are suggested for clear cell and endometrioid
ovarian carcinomas respectively [90-92]. Besides, IP3k/AKT/
mTOR is the most investigating signaling mechanism for many
cellular activities like cell growth, cell survival, proliferation, protein
synthesis, transcription, and angiogenesis. Dysregulation of the
signaling pathway causes activation of other downstream signaling
mechanisms leading oncogenesis in humans. IP3K is a lipid kinase
of G-protein coupled receptor and receptor tyrosine kinase (RTKs)
family protein according to their structure; it is categorized in three
classes I, II, and III. Again, Class, I of IP3K is sub-classified into two
categories (IA and IB).
It is a group of heterodimer proteins consisting of p85 and p110
regulatory subunit encoded in 3 distinct genes viz; PIK3CA, PIK3CB,
and PIK3CD. Catalytic activation of p110 leads to phosphorylation
of 3rd carbon of the inositol that had of phosphatidylinositol 4-5
biphosphate (PIP2) lead to produce phosphatidylinositol [3-5]
triphosphate (PIP3), for the activation of 2nd messenger PIP3,
thus triggering AKT in the plasma membrane. The blockade
of this pathway leads to independent cell growth due to a lack
of a negative regulator of PIP3K/AKT/ [93,94]. Endometriosis
is a benign inflammatory disease however, the molecular and
cellular features work in a similar manner like; malignancy
occurs, including increased cell proliferation, re-expression of
the pluripotent transcription factor OCT4 [95], epithelial-tomesenchymal
transition [96] acquisition of migratory phenotype
[95,97] development of distant foci, cell adhesion, invasion [98]
angiogenesis, and at times, treatment resistance [99]. It is not fully
declined but there is a strong association between endometriosis
and ovarian cancer. Genetic mutation is the major factor of
ovarian cancer thus many protein markers are used for advanced
diagnosis, prognosis, and treatment. All genomic and proteomic
markers facilitate the development of the best diagnostic tool for
endometriosis - associated ovarian cancer.
Management
Endometriosis is a chronic inflammatory disease that
requires long term or lifelong treatment. Three major therapeutic
management types exist for endometriosis, including Medical
treatment, surgery, and assisted reproductive technology (ART)
[100]. Accurate diagnosis is imperative because it provides outright
information regarding severity of the disease. Many societies
such as American College of Obstetricians and Gynecologists and
American Societies of Reproductive Medicine approved medical
treatment before definitive diagnosis [101,102]. The medical
treatment provides only short term pain relief but when combined
with surgical intervention, divulges long term relief. The main
concern is how endometriosis-associated pain can be managed.
Physicians believe in optimal management for this condition. All
these treatments are suppressive and do not possess curative effect.
1) Egg preservation in young patients affected by
endometriosis.
2) Preoperative surgical management to inhibit evolution
and to avoid removal of cyst that might look like endometriosis.
3) Postoperative hormonal suppression to decrease
recurrence of endometriosis [103].
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Non-steroidal anti-inflammatory drugs (NSAIDs) are used to reduce pain associated with dysmenorrhea in 80% of women. It inhibits cyclooxygenase (COX), a key enzyme in the biosynthesis of prostaglandin in endometriosis-associated pain and inflammation. Cyclooxygenase is two types viz; COX-1 and COX-2. NSAIDs and acetylsalicylic acid (ASA) inhibit both COX-1 and COX-2 but have a selective effect to primarily inhibit COX-2. However, management with NSAIDs is not significant, because endometrial pain appears by involving other factors in addition to prostaglandin. Ibuprofen, Naproxen, Mefenamic acid, Diclofenac, Meclofenamate, Ketoprofen are commonly seen effective in dysmenorrhea. It acts quickly within 30 to 60 minutes and can be taken on a regular basis according to the score of pain. Oral contraceptives can be used as secondline treatment if NSAIDs fail or decrease the pain incompletely. Individual NSAIDs have different types of actions depending upon their selective and non-selective receptor binding capacity. Besides, there might be specific side effects of NSAIDs, such as gastrointestinal (nausea, vomiting, peptic ulcer), hypersensitivity, rays syndrome, intestinal nephritis, etc. [104-106].
Hormonal Therapy
Gonadotropin-releasing hormone (GnRH) agonists are useful to deplete further synthesis of endogenous gonadotropins from the pituitary gland, interrupting the menstrual cycle, eventually resulting to cause endometrial atrophy and amenorrhea [107]. Progestin is an acceptable alternative drug for first-line therapy of endometriosis and has been used for over 30 years. Endometrial carcinoma has been treated with a more diverse method than the other malignant tumors that affect intra pelvic organs in untreatable cases. Radiotherapy, chemotherapy, and hormonal therapy are used in cases of endometrial malignancy. Here in hormonal treatment is better for those who wish to remain fertile after the early development of pre-menopausal endometrial cancer [108,109]. Progestin is effective in suppressing endometrial tumor growth in comparison to GnRH and Danazol. The cost-effectiveness and lower incidence of estrogen deficiency associated with adverse drug reaction (ADR) are lower than GnRH and danazol in the treatment of endometriosis. Thus, investigators have considered progestin as more effective and useful than GnRH and Danazol. Progestin with hormonal contraceptives can be provided either alone or in combination with estrogen to prevent development endometriosis hyperplasia. It decreases glandular cellularity by including apoptosis and inhibits angiogenesis in the myometrium [109-111].
Medroxyprogestrone Acetate (MPA)
MPA is a synthetic and one of the most studied progestin that is typically used to cure irregular menstruation and abnormal uterine bleeding. It has been a greater effect on pain and improving quality life in placebo therapy, but the MPA and GnRH agonist dose 15-50 mg daily administration the efficacy has been found equivalent [110,112]. Ushijima K et al. conducted the Multicenter phase II clinical trial study. They found an 82% complete rate response in endometrial hyperplasia, where all the patients received 600 mg MPA with low dose Aspirin for 8 to 16 weeks and were monitored in following after 3 years. During the observation period, 12 pregnancies and 7 normal deliveries were achieved, which proved that MPA is effective in fertility-sparing treatment with a high dose and least toxicities for endometrial cancer and endometrial hyperplasia [113].
Danazol
It is a synthetic steroid with strong anti-gonadotropins
and weak androgenic properties, which inhibit estrogen and
progesterone receptors’ results in preventing endometriotic
atrophy and reduced menstrual cycles [114]. Selak V, et al. 2001
in a Cochrane systematic review, evaluated the effectiveness of
Danazol to placebo in the treatment of endometriosis along with
infertility in women of reproductive age. The data represented that
treatment with Danazol was effective in endometriosis-associated
pain, and Laparoscopic scores were improved as compared to with
or without placebo treatment [115]. Luisi S, et al. 2009 evaluated
the efficacy of Danazol in young women with menorrhagia. Total
55 (30 had one or two miscarriages & 25 were nullipara) women
were selected for this study after biopsies (hysteroscopy-directed
biopsy) to exclude endometriosis hyperplasia, polyps. Also, they
were prescribed 200 mg danazol daily at night by vaginal route for
6 months.
The result was useful as there was a significant reduction in
the severity of blood loss in all women after 2 months of treatment
[116]. Furthermore, another investigation by Szubert M, et al.
2014 depicted the efficacy of danazol treatment in endometriosis
and pain management. 103 cases were selected and laparoscopy
was performed during follow up. Only 71 women were diagnosed
with having endometriosis and other women were suffering from
minor pain in the pelvis. Treated with 2X200 mg danazol daily for
6 months, only 35 participants completed the study (two follow up
visits at third and sixth months). After treatment, the pain score
and concentration of CA-125 in plasma was significantly decreased
[117].
Hormonal Contraceptives
Limited studies said that oral contraceptives are useful in the treatment of endometriosis-associated with chronic pain. GnRH is the principal regulatory hormone of FSH) and LH. All these hormones are involved in the synthesis and regulation of estrogen for ovulation and follicular development. Oral Contraceptive Pill (OCP) inhibits the synthesis of LH and FSH to prevent estrogen and progesterone synthesis by suppressing the activation of GnRH, resulting in the prevention of endometrial proliferation and produces scanty cervical mucus [109]. Estrogen - progestin combination or alone progestin is one of the most common contraceptives used as the first-hand treatment for chronic pain the in case of endometriosis and ovarian function. These can be prescribed through oral contraceptive pills, transdermal patches, vaginal ringsEstrogen - progestin combination or alone progestin is one of the most common contraceptives used as the first-line treatment of endometriosis-associated with chronic pain and ovarian function. These can be prescribed through oral contraceptive pills, transdermal patches, vaginal rings [118]. According to the degree of success in women who were affected by endometriosis or its associated pain, the cost, ease of administration, and tolerance effect are the key factor of these drugs to make them common.
Continuous therapy of combined oral contraceptives (COC) is better to control chronic pain as compared to cyclic administration [111]. A systemic review by Vercellini P, et al. 2011 revealed that the common relative risk of 0.63 [95% confidence interval (CI), 0.47- 0.85] for current OC users, 1.21 (95% CI, 0.94-1.56) for past users and 1.19 (95% CI, 0.89-1.60) forever users. This study indicated that OC is effective in reducing endometriosis and its symptoms [119]. A study undergone by Sullivan H, et al. 1999 to rule out whether the dose of COC was effective in endometriosis or ovarian activities revealed that COC had positive impacts on endometriosis. A total of 30 healthy women were included, who were administered 60 mcg of Gestodene and 15 mcg of Ethinyl Estradiol on 21st and 24th day in every 28 days menstrual cycle and were monitored over 5 months. The outcome was that such a regimen is an active treatment for endometriosis as compared to an alone ultra-low dose of estrogen treatment [120]. Medroxyprogesterone acetate 10-100 mg of norethindrone acetate 5 mg daily is commonly prescribed to treat endometriosis; this progesterone is completely suppressing hypothalamic-pituitary axis for preventing ovulation [121].
Gonadotropin Releasing Hormone (GnRH) Agonist
GnRH agonists are one of the most common drugs used for the treatment of endometriosis. These drugs are down-regulating GnRH receptors from the hypothalamic-pituitary gland, which decreases gonadotropin secretion to inhibit ovulation and reduce serum estrogen level [122]. It is prescribed only after consultation with a physician. Leuprolide is a GnRH agonist that is effective in endometriosis as approved by the Food Drug Administration (FDA) [123]. Initially, the administration of Leuprolide stimulates LH and FSH which leads to increment in steroidogenesis thus, it elevates estrogen level in females and testosterone along with dihydrotestosterone (DHT) in males. Moreover, long term administration of Leuprolide can decrease the hormonal level, which may ultimately inhibit gonadotropin release by inhibiting the secretion of LH and FSH in both males and females [124]. In comparison with combined hormonal contraceptive (CHC) treatment, GnRH agonists have better efficacy in endometriosis and its associated chronic pain [125]. Various research have been conducted to improve novel treatment with GnRH agonists for endometriosis and its associated symptoms, to conclude how currently existing therapies work, which type of biochemical abnormalities are seen and how can they be minimized. All these studies concluded a similar effect of GnRH agonists found effective in preventing endometriosis and chronic pain [126-130].
Gonadotropin Releasing Hormone Antagonist (GnRHAnta.)
GnRH antagonists have an advantage over the agonists about initial flare and hypoestrogenism through a direct mechanism that completely inhibits the GnRH receptors resulting in immediate suppression of LH and FSH secretion [131]. With the aid of cloning and high throughput peptide screening of oral human GnRH receptors, antagonist Elagolix was developed. It has a high affinity to bind GnRH receptors and reduces the interaction with CYP450 in inhibiting LH and FSH [132,133]. Clinical research was conducted in two different doses; 150 and 200 mg. First was 150mg daily for 24 months and second, 200 mg twice a day for 6 months. In 2018, USFDA approved 150 - 200 mg Elagolix for the management of endometriosis [134,135]. At the moment, various formulations are available but oral, and injectable preparations are widely used that competitively inhibit the GnRH secretion. The other two types of GnRH antagonists called Relugolix and Linzagolix are introduced, which work to prevent the advanced stage of endometriosisassociated pain. Both the drugs are under clinical trials phase III. In the clinical trial, 40 mg Linzagolix is used in combination with Ethyl estradiol 0.1 mg or 0.5 mg NETA (clinical trial registration No. NCT03204318, NCT03204331, and NCT02778919) [135].
Aromatase Inhibitor
The Aromatase enzyme comprised two types of polypeptides. The first one belongs to the superfamily of the cytochrome P450 (CYP450arom), which is the product of single gene CYP19, and second is flavoprotein (flavoprotein NADPH-CYP450 reductase). It is expressed on different body sites like ovarian granulosa cells, adipose tissue, placental syncytiotrophoblast, osteoblasts, and brain. Aromatase’s main source is ovarian cells in premenopausal women, while adipose tissues in postmenopausal women [136,137]. Aromatase converts approximately 2% of Androstenedione to E1. Further, it turned to E2 in postmenopausal women by 17ß-Hydroxysteroid dehydrogenase type 1 in peripheral tissue resulting to high level of E2 circulating in the blood to cause endometrial hyperplasia and carcinoma [138]. The agent Aromatase inhibitor (AIs) was first employed to manage estrogen receptor activating breast carcinoma.
AIs represent the new promising treatment of endometriosis. It has the capability to reduce estrogen production by inhibiting the enzyme cytochrome P450 [139]. AIs are classified into three generations. The first generation is Glutehimide, which has many adverse effects like lethargy, hypersensitivity, nausea, etc. The second generation is, Fadrozole and Formestancel have more selective and higher efficacy over the first generation and have less adverse effects. The third generation Aromatase inhibitors, namely; Letrozole, Anastrazole, and Examestande, are triazole derivatives that are selective, reversible as well as more potent, making them ideal for using clinically. These agents are prescribed in premenopausal women to decrease the estrogen level and increase FSH secretion from pituitary glands. Treatment with Aromatase inhibitors must be combined with other potent drugs to downregulate [137].
Letrozole
Letrozole is a non-steroidal aromatase inhibitor that has been commonly used for the management of endometriosis-associated pelvic pain and breast carcinoma. It was first reported in 2004 for the management of endometriosis. Letrozole has been used alone or in combination with other steroidal or non-steroidal analogs for the treatment of [140]. In the preclinical study, the animals were selected and made develop endometriosis by Vernon and Wilson’s method. After the 14th day of model development, the animals underwent laparoscopy to measure heterotrophic. After that, they were provided 0.5 mg/kg AIs (Letrozole) daily for 3 weeks. Upon completion of treatment, the animals were re-examined, and the size of heterotrophy was determined. Besides, the histopathology study was carried out in both groups (control and AIs treated group). The result suggested that 0.5 mg/kg for 21 days of treatment with Letrozole decreases the size of heterotopies. The total percentage of reduction of 79.92% ± 7.89% was observed [141]. A Prospective non-randomized study was carried out, including 20 patients with endometriosis and its associated chronic pelvic pain. Both Letrozole and norethindrone were prescribed in combined form as 2.5mg/kg for 6 months starting from the 3rd day of the menstrual cycle. Besides, the pain score was recorded 30 days before starting the treatment. The result showed that following the procedure, and all the patients showed significant improvement in pain and [142].
Role of Micro-RNAs in Endometriosis Treatment
Micro-RNAs (miRNAs) are the highly conserved 9-22 nucleotide long non-coding RNAs that play a crucial role in cellular functions such as; cell proliferation, angiogenesis, apoptosis, and gene expression. miRNAs are directly bound to the 3 untranslated regions of messenger RNA (mRNA [143]. miRNAs have been found in most body fluids and the expression of miRNAs differs between healthy and diseased people however its physiological roles are obscure [144]. Endometriosis is a multifactorial disease characterized by the presence of endometrial tissue surrounding the womb. miRNAs are expressed in different gynecological disorders like; malignancies, leiomyoma, endometriosis, etc. Ovarian carcinoma is a rampant lethal malignancy in developed countries [145]. As angiogenesis is a key factor in endometriosis, several studies have reported that increment in VEGF level causes endometriosis [146-148]. miRNAs are involved in apoptosis disease progression as a result of which, in endometriosis, they are used as a diagnosing tool for detecting the progress of the disease and cell proliferation. Also, miRNAs are potential and efficient in the early detection of [143].
Anti-Angiogenesis Factor
Presently available medical treatment is effective to suppress
hormonal synthesis. Oral contraceptives, androgenic agents,
progestin analogs, GnRH agonists and GnRH antagonists have
been successfully used for the treatment of endometriosis.
However, these agents are not effective in eradicating the disease
in some cases. Long term use of these agents exhibits major
adverse effects. Interleukins-8, vascular endothelial growth
factor (VGEF) receptor-2, Endoglin, Urokinase-type plasminogen
activator, matrix metalloproteinase-2, and 9, as well as a placental
growth factor, is found in an activated endometrial cell in tumors
[111,149-151]. Previous studies showed that soluble truncated
fms like tyrosine kinase-1 receptor and affinity-purified VEGF
antibody are significantly useful to inhibit the angiogenesis
factor in endometriosis. The blockade of VEGF signaling prevents
endometrial lesions and associated carcinoma. Bevacizumab is
a recombinant humanized anti-VEGF monoclonal antibody that
prevents vascular density and also leads increment in apoptotic cell
demise in case of surgically induced endometriosis.
Bevacizumab is in phase II clinical trial 15 mg/kg intravenous
route of administration every 3 weeks until the disease progression
is effective in endometrial carcinoma [152,153]. Thalidomide is also
used as an anti-angiogenic agent in endometriosis. The mechanism
of action of thalidomide is suppression of phosphoinositide 3 kinases
(P13K)/protein kinase B (AKT) signaling to inhibit the formation of
angiogenesis-related effect [154]. Antônio LG, et al. investigated the
effect of thalidomide in the progression of endometrial lesions. 1
and 10 mg/kg thalidomide was administered in Wistar rats daily
for 10 days. After 10 days of treatment, the tissue was isolated,
and the cell proliferation index (CPI) was identified. The result
proved that thalidomide had significantly reduced the lesion area
and CPI [155]. Women who had relapsing endometriosis after
surgical treatment of ovarian and peritoneal endometriosis were
made discontinue GnRH agonist treatment, meanwhile, 300 mg/kg
of thalidomide was prescribed for 6 months. Eventually, following
the anti-angiogenic treatment with thalidomide, the relapse of
endometriosis was completely suppressed [156].
Surgical Treatment
Surgical management of endometriosis requires extensive knowledge of the disease that should be managed by a multidisciplinary team to lead professional gynecologists. Laparoscopy is a useful tool for the diagnosis of disease and determining the severity of disease before starting the surgical treatment [157]. It is effective in suppressing symptoms and chronic pain and can increase the fertility of women. Approximately, 50% of cases will re-develop symptoms within a few years of surgery. The severity of the disease determines the successful surgical treatment of Endometriosis [122]. Patients who have chronic pelvic pain or ovarian endometrium and do not respond to medical management need surgical treatment. There are some approaches for considering surgical management viz;
1) Egg preservation in young patients affected by
endometriosis.
2) Preoperative surgical management to inhibit evolution
and to avoid removal of cyst that might look like endometriosis.
3) Postoperative hormonal suppression to decrease
recurrence of endometriosis [103].
Conclusion
It is mandatory to understand that endometriosis is a complex, debilitating disease comprising multiple factors in its origin and development. Researchers are comprehending the distinct clinical features, including chronic pelvic pain and infertility, to discover the significant markers or therapeutic strategies to battle the mysterious disease. It is significant to gain cognizance into the complex etiology of endometriosis due to different causes viz; the unavailability of non-invasive diagnostic markers, delay in diagnosis, high risk of recurrence of the disease following surgical removal of the tissue, and lack of a definitive cure for the disease. This article will furnish the readers with up-to-date knowledge regarding the pathogenesis, biomarkers, association of infertility, pregnancy, and carcinoma with the ailment and also shed light on the management of the disease.
Future Prospective
Still, endometriosis research is lacking the exact mechanism for etiology, biomarkers, and effective treatment modalities to generate convincing data with high sensitivity and specificity. Besides, limitations derive from small sample size and suboptimal characterization of specimens.
Acknowledgement
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript. We would like to thank Mrs. Sabita shah for her great contribution in editing the manuscript.
Ethical Consideration
Conflict of interest: Authors declare that they have no conflict of interest.
References
- Giudice LC (2010) Clinical practice. Endometriosis. The New England journal of medicine 362(25): 2389-2398.
- Klemmt PAB, Starzinski Powitz A (2018) Molecular and Cellular Pathogenesis of Endometriosis. Current women's health reviews 14(2): 106-116.
- Giudice LC, Kao LC (2004) Endometriosis. Lancet 364(9447): 1789-1799.
- Koger KE, Shatney CH, Hodge K, Mc Clenathan JH (1993) Surgical scar endometrioma. Surgery, gynecology & obstetrics 177(3): 243-246.
- Macer ML, Taylor HS (2012) Endometriosis and infertility: a review of the pathogenesis and treatment of endometriosis-associated infertility. Obstetrics and gynecology clinics of North America 39(4): 535-549.
- Machairiotis N, Stylianaki A, Dryllis G, Zarogoulidis P, Kouroutou P, et al. (2013) Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagnostic pathology 8: 194.
- Burghaus S, Fehm T, Fasching PA, Blum S, Renner SK, et al. (2016) The International Endometriosis Evaluation Program (IEEP Study) - A Systematic Study for Physicians, Researchers and Patients. Geburtshilfe und Frauenheilkunde 76(8): 875-881.
- Liu K, Zhang W, Liu S, Dong B, Liu Y (2015) Hepatic endometriosis: a rare case and review of the literature. European journal of medical research 20(1): 48.
- Agarwal N, Subramanian A (2010) Endometriosis - morphology, clinical presentations and molecular pathology. Journal of laboratory physicians 2(1): 1-9.
- Olive DL, Schwartz LB (1993) Endometriosis. The New England journal of medicine 328(24): 1759-1769.
- Stilley JA, Birt JA, Sharpe Timms KL (2012) Cellular and molecular basis for endometriosis-associated infertility. Cell and tissue research 349(3): 849-862.
- Eskenazi B, Warner ML (1997) Epidemiology of endometriosis. Obstetrics and gynecology clinics of North America 24(2): 235-258.
- Allaire C (2006) Endometriosis and infertility: a review. The Journal of reproductive medicine 51(3): 164-168.
- Somigliana E, Vigano P, Rossi G, Carinelli S, Vignali M, et al. (1999) Endometrial ability to implant in ectopic sites can be prevented by interleukin-12 in a murine model of endometriosis. Human reproduction (Oxford, England) 14(12): 2944-2250.
- Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, et al. (2016) A prospective cohort study of endometriosis and subsequent risk of infertility. Human reproduction (Oxford, England). 2016;31(7):1475-1482.
- (1996) Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertility and sterility 67(5): 817-821.
- Tanbo T, Fedorcsak P (2017) Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta obstetricia et gynecologica Scandinavica 96(6): 659-667.
- Adamson GD, Pasta DJ (2010) Endometriosis fertility index: the new, validated endometriosis staging system. Fertility and sterility 94(5): 1609-1615.
- Bulletti C, Coccia ME, Battistoni S, Borini A (2010) Endometriosis and infertility. Journal of assisted reproduction and genetics 27(8): 441-447.
- Fourquet J, Sinaii N, Stratton P, Khayel F, Alvarez Garriga C, et al. (2015) Characteristics of women with endometriosis from the USA and Puerto Rico. Journal of endometriosis and pelvic pain disorders 7(4): 129-135.
- Alimi Y, Iwanaga J, Loukas M, Tubbs RS (2018) The Clinical Anatomy of Endometriosis: A Review. Cureus 10(9): e3361.
- As Sanie S, Black R, Giudice LC, Gray Valbrun T, Gupta J, et al. (2019) Assessing research gaps and unmet needs in endometriosis. American journal of obstetrics and gynecology 221(2): 86-94.
- Rogers PA, D'Hooghe TM, Fazleabas A, Giudice LC, Montgomery GW, et al. (2013) Defining future directions for endometriosis research: workshop report from the 2011 World Congress of Endometriosis In Montpellier, France. Reproductive sciences 20(5): 483-499.
- Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, et al. (2012) The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Human reproduction (Oxford, England) 27(5): 1292-1299.
- Guo SW (2009) Recurrence of endometriosis and its control. Human reproduction update 15(4): 441-461.
- Simpson PD, Mc Laren JS, Rymer J, Morris EP (2015) Minimising menopausal side effects whilst treating endometriosis and fibroids. Post reproductive health 21(1): 16-23.
- Simoens S, Hummelshoj L, D'Hooghe T (2007) Endometriosis: cost estimates and methodological perspective. Human reproduction update 13(4): 395-404.
- De Graaff AA, D'Hooghe TM, Dunselman GA, Dirksen CD, Hummelshoj L, et al. (2013) The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Human reproduction (Oxford, England) 28(10): 2677-2685.
- Pritts EA, Taylor RN (2003) An evidence-based evaluation of endometriosis-associated infertility. Endocrinol Metab Clin North Am 32(3): 653-667.
- Vessey MP, Villard Mackintosh L, Painter R (1993) Epidemiology of endometriosis in women attending family planning clinics. BMJ 306(6871): 182-184.
- Bougie O, Yap MI, Sikora L, Flaxman T, Singh S (2019) Influence of race/ethnicity on prevalence and presentation of endometriosis: a systematic review and meta-analysis. BJOG 126(9): 1104-1115.
- Cramer DW, Missmer SA (2002) The epidemiology of endometriosis. Annals of the New York Academy of Sciences 955(1): 11-22.
- Missmer SA, Cramer DW (2003) The epidemiology of endometriosis. Obstetrics and gynecology clinics of North America 30(1): 1-19.
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, et al. (2004) Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 160(8): 784-796.
- Cramer DW, Wilson E, Stillman RJ, Berger MJ, Belisle S, et al. (1986) The relation of endometriosis to menstrual characteristics, smoking, and exercise. Jama 255(14): 1904-1908.
- Darrow SL, Vena JE, Batt RE, Zielezny MA, Michalek AM, et al. (1993) Menstrual cycle characteristics and the risk of endometriosis. Epidemiology (Cambridge, Mass) 4(2):135-142.
- Fortin M, Lepine M, Page M, Osteen K, Massie B, et al. (2003) An improved mouse model for endometriosis allows noninvasive assessment of lesion implantation and development. Fertil Steril 80(Suppl 2): 832-838.
- Bruner Tran KL, Webster Clair D, Osteen KG (2002) Experimental endometriosis: the nude mouse as a xenographic host. Annals of the New York Academy of Sciences 955: 328-39; discussion 340-342, 396-406.
- Edwards AK, Nakamura DS, Virani S, Wessels JM, Tayade C (2013) Animal models for anti-angiogenic therapy in endometriosis. Journal of reproductive immunology 97(1): 85-94.
- Lagana AS, Vitale SG, Salmeri FM, Triolo O, Ban Frangez H, et al. (2017) Unus pro omnibus, omnes pro uno: A novel, evidence-based, unifying theory for the pathogenesis of endometriosis. Medical hypotheses 103: 10-20.
- Ozkan S, Murk W, Arici A (2008) Endometriosis and infertility: epidemiology and evidence-based treatments. Annals of the New York Academy of Sciences 1127: 92-100.
- Chegini N, Roberts M, Ripps B (2003) Differential expression of interleukins (IL)-13 and IL-15 in ectopic and eutopic endometrium of women with endometriosis and normal fertile women. American journal of reproductive immunology 49(2): 75-83.
- Hapangama DK, Turner MA, Drury JA, Quenby S, Saretzki G, et al. (2008) Endometriosis is associated with aberrant endometrial expression of telomerase and increased telomere length. Human reproduction 23(7): 1511-1519.
- Nap AW, Groothuis PG, Demir AY, Evers JL, Dunselman GA (2004) Pathogenesis of endometriosis. Best practice & research Clinical obstetrics & gynaecology 18(2): 233-244.
- Sasson IE, Taylor HS (2008) Stem cells and the pathogenesis of endometriosis. Annals of the New York Academy of Sciences 1127: 106-115.
- Izawa M, Taniguchi F, Terakawa N, Harada T (2013) Epigenetic aberration of gene expression in endometriosis. Frontiers in bioscience 5: 900-910.
- Wu Y, Halverson G, Basir Z, Strawn E, Yan P, et al. (2005) Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. American journal of obstetrics and gynecology 193(2): 371-380.
- Koike N, Higashiura Y, Akasaka J, Uekuri C, Ito F, et al. (2015) Epigenetic dysregulation of endometriosis susceptibility genes (Review). Molecular medicine reports 12(2): 1611-1616.
- Bjorkman M, Malterud K (2009) Lesbian women's experiences with health care: a qualitative study. Scandinavian journal of primary health care 27(4): 238-243.
- Holoch KJ, Lessey BA (2010) Endometriosis and infertility. Clinical obstetrics and gynecology 53(2): 429-438.
- Cahill DJ, Hull MG (2000) Pituitary-ovarian dysfunction and endometriosis. Human reproduction update 6(1): 56-66.
- Hughes EG, Fedorkow DM, Collins JA (1993) A quantitative overview of controlled trials in endometriosis-associated infertility. Fertility and sterility 59(5): 963-970.
- Evans MB, Decherney AH (2017) Fertility and Endometriosis. Clinical obstetrics and gynecology 60(3): 497-502.
- Doody MC, Gibbons WE, Buttram VC (1988) Linear regression analysis of ultrasound follicular growth series: evidence for an abnormality of follicular growth in endometriosis patients. Fertility and sterility 49(1): 47-51.
- Garrido N, Pellicer A, Remohí J, Simón C (2003) Uterine and ovarian function in endometriosis. Seminars in reproductive medicine 21(2): 183-192.
- Hahn DW, Carraher RP, Foldesy RG, Mc Guire JL (1986) Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. American journal of obstetrics and gynecology 155(5): 1109-1113.
- Tanbo T, Omland A, Dale PO, Abyholm T (1995) In vitro fertilization/embryo transfer in unexplained infertility and minimal peritoneal endometriosis. Acta obstetricia et gynecologica Scandinavica 74(7): 539-543.
- Guzick DS, Silliman NP, Adamson GD, Buttram VC, Canis M, et al. (1997) Prediction of pregnancy in infertile women based on the American Society for Reproductive Medicine's revised classification of endometriosis. Fertil Steril 67(5): 822-829.
- D'Hooghe TM, Debrock S, Hill JA, Meuleman C (2003) Endometriosis and subfertility: is the relationship resolved? Seminars in reproductive medicine 21(2): 243-254.
- Porpora MG, Tomao F, Ticino A, Piacenti I, Scaramuzzino S, et al. (2020) Endometriosis and Pregnancy: A Single Institution Experience. International journal of environmental research and public health 17(2): 401.
- Lalani S, Choudhry AJ, Firth B, Bacal V, Walker M, et al. (2018) Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Human reproduction 33(10): 1854-1865.
- Stephansson O, Kieler H, Granath F, Falconer H (2009) Endometriosis, assisted reproduction technology, and risk of adverse pregnancy outcome. Human reproduction 24(9): 2341-2347.
- Collinet P, Fritel X, Revel Delhom C, Ballester M, Bolze PA, et al. (2018) Management of endometriosis: CNGOF/HAS clinical practice guidelines - Short version. Journal of gynecology obstetrics and human reproduction 47(7): 265-274.
- (2012) Practice Committee of the American Society for Reproductive Medicine, Endometriosis and infertility: a committee opinion. Fertility and sterility 98(3): 591-598.
- Bazot M, Darai E (2017) Diagnosis of deep endometriosis: clinical examination, ultrasonography, magnetic resonance imaging, and other techniques. Fertility and sterility 108(6): 886-894.
- Nisolle M, Donnez J (1997) Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertility and sterility 68(4): 585-596.
- Biscaldi E, Ferrero S, Fulcheri E, Ragni N, Remorgida V, et al. (2007) Multislice CT enteroclysis in the diagnosis of bowel endometriosis. European radiology 17(1): 211-219.
- Biscaldi E, Ferrero S, Leone Roberti Maggiore U, Remorgida V, Venturini PL, et al. (2014) Multidetector computerized tomography enema versus magnetic resonance enema in the diagnosis of rectosigmoid endometriosis. European journal of radiology 83(2): 261-267.
- Fassbender A, Burney RO, O DF, D'Hooghe T, Giudice L (2015) Update on Biomarkers for the Detection of Endometriosis. BioMed research international 2015: 130854.
- Rogers PA, D'Hooghe TM, Fazleabas A, Gargett CE, Giudice LC, et al. (2009) Priorities for endometriosis research: recommendations from an international consensus workshop. Reproductive sciences 16(4): 335-346.
- Bhyan SB, Zhao L, Wee Y, Liu Y, Zhao M (2019) Genetic links between endometriosis and cancers in women. PeerJ 7: e8135.
- Yamamoto Y, Wakikawa A, Ueno A, Nagai R, Matsumoto M, et al. (2018) Comparison of endometriotic cysts and ovarian cancer in association with endometriotic cysts. Cancer treatment and research communications 14: 26-29.
- Sampson JA (1927) Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. The American journal of pathology 3(2): 93-110.
- Kalaitzopoulos DR, Mitsopoulou A, Iliopoulou SM, Daniilidis A, Samartzis EP, et al. (2020) Association between endometriosis and gynecological cancers: a critical review of the literature. Archives of Gynecology and Obstetrics 301(2): 355-367.
- Melin A, Sparén P, Persson I, Bergqvist A (2006) Endometriosis and the risk of cancer with special emphasis on ovarian cancer. Human reproduction 21(5): 1237-1242.
- Dawson A, Fernandez ML, Anglesio M, Yong PJ, Carey MS (2018) Endometriosis and endometriosis-associated cancers: new insights into the molecular mechanisms of ovarian cancer development. Ecancermedicalscience 12: 803.
- Wei JJ, William J, Bulun S (2011) Endometriosis and ovarian cancer: a review of clinical, pathologic, and molecular aspects. International journal of gynecological pathology : official journal of the International Society of Gynecological Pathologists 30(6): 553-568.
- Nezhat F, Datta MS, Hanson V, Pejovic T, Nezhat C, et al. (2008) The relationship of endometriosis and ovarian malignancy: a review. Fertility and sterility 90(5): 1559-1570.
- Jiang X, Hitchcock A, Bryan EJ, Watson RH, Englefield P, et al. (1996) Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumor suppressor gene loci. Cancer research 56(15): 3534-3539.
- Torng PL (2017) Clinical implication for endometriosis associated with ovarian cancer. Gynecology and minimally invasive therapy 6(4):152-156.
- Guo SW (2020) Cancer-associated mutations in endometriosis: shedding light on the pathogenesis and pathophysiology. Human Reproduction Update 26(3): 423-449.
- Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, et al. (2000) Loss of heterozygosity on 10q23. 3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer research 60(24): 7052-7056.
- Worley MJ, Welch WR, Berkowitz RS, Ng SW (2013) Endometriosis-associated ovarian cancer: a review of pathogenesis. International journal of molecular sciences 14(3): 5367-5379.
- Mandai M, Yamaguchi K, Matsumura N, Baba T, Konishi I (2009) Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. International journal of clinical oncology 14(5): 383-391.
- Wiegand KC, Shah SP, Al Agha OM, Zhao Y, Tse K, et al. (2010) ARID1A mutations in endometriosis-associated ovarian carcinomas. The New England journal of medicine 363(16): 1532-1543.
- Jones S, Wang TL, Shih Ie M, Mao TL, Nakayama K, et al. (2010) Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330(6001): 228-231.
- Yanaihara N, Caplen N, Bowman E, Seike M, Kumamoto K, et al. (2006) Unique microRNA molecular profiles in lung cancer diagnosis and prognosis. Cancer cell 9(3): 189-198.
- Kolasa IK, Rembiszewska A, Janiec Jankowska A, Dansonka Mieszkowska A, Lewandowska AM, et al. (2006) PTEN mutation, expression and LOH at its locus in ovarian carcinomas. Relation to TP53, K-RAS and BRCA1 mutations. Gynecologic oncology 103(2): 692-697.
- Palacios J, Gamallo C (1998) Mutations in the beta-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer research 58(7): 1344-1347.
- Banz C, Ungethuem U, Kuban RJ, Diedrich K, Lengyel E, et al. (2010) The molecular signature of endometriosis-associated endometrioid ovarian cancer differs significantly from endometriosis-independent endometrioid ovarian cancer. Fertility and sterility 94(4): 1212-1217.
- Madore J, Ren F, Filali Mouhim A, Sanchez L, Köbel M, et al. (2010) Characterization of the molecular differences between ovarian endometrioid carcinoma and ovarian serous carcinoma. The Journal of pathology 220(3): 392-400.
- Stany MP, Vathipadiekal V, Ozbun L, Stone RL, Mok SC, et al. (2011) Identification of novel therapeutic targets in microdissected clear cell ovarian cancers. PloS one 6(7): e21121.
- Ediriweera MK, Tennekoon KH, Samarakoon SR (2019) Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Seminars in cancer biology 59: 147-160.
- Gasparri ML, Bardhi E, Ruscito I, Papadia A, Farooqi AA, et al. (2017) PI3K/AKT/mTOR pathway in ovarian cancer treatment: are we on the right track? Geburtshilfe und Frauenheilkunde 77(10): 1095-1103.
- Au HK, Chang JH, Wu YC, Kuo YC, Chen YH, et al. (2015) TGF-betaI Regulates Cell Migration through Pluripotent Transcription Factor OCT4 in Endometriosis. PloS one 10(12): e0145256.
- Bartley J, Julicher A, Hotz B, Mechsner S, Hotz H (2014) Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Archives of gynecology and obstetrics 289(4): 871-881.
- Ramakrishnan M, Mathur SR, Mukhopadhyay A (2013) Fusion-derived epithelial cancer cells express hematopoietic markers and contribute to stem cell and migratory phenotype in ovarian carcinoma. Cancer research 73(17): 5360-5370.
- Young VJ, Brown JK, Saunders PT, Horne AW (2013) The role of the peritoneum in the pathogenesis of endometriosis. Human reproduction update 19(5): 558-569.
- Cakmak H, Taylor HS (2010) Molecular mechanisms of treatment resistance in endometriosis: the role of progesterone-hox gene interactions. Seminars in reproductive medicine 28(1): 69-74.
- Chapron C, Marcellin L, Borghese B, Santulli P (2019) Rethinking mechanisms, diagnosis and management of endometriosis. Nature Reviews Endocrinology 15(11): 666-682.
- (2010) Practice bulletin no. 114: management of endometriosis. Obstet Gynecol 116(1): 223-236.
- Practice Committee of the American Society for Reproductive Medicine (2014) Treatment of pelvic pain associated with endometriosis: a committee opinion. Fertility and sterility 101(4): 927-935.
- Nezhat C, Vang N, Tanaka PP, Nezhat C (2019) Optimal Management of Endometriosis and Pain. Obstetrics & Gynecology 134(4): 834-839.
- Frackiewicz EJ (2000) Endometriosis: an overview of the disease and its treatment. Journal of the American Pharmaceutical Association (Wash) 40(5): 645-657.
- Ruoff G, Lema M (2003) Strategies in pain management: new and potential indications for COX-2 specific inhibitors. Journal of pain and symptom management 25(2 Suppl): 21-31.
- Sostres C, Gargallo CJ, Arroyo MT, Lanas A (2010) Adverse effects of non-steroidal anti-inflammatory drugs (NSAIDs, aspirin and coxibs) on upper gastrointestinal tract. Best practice & research Clinical gastroenterology 24(2): 121-132.
- Giudice LC (2010) Endometriosis. New England Journal of Medicine 362(25): 2389-2398.
- Banno K, Kisu I, Yanokura M, Tsuji K, Masuda K, et al. (2012) Progestin therapy for endometrial cancer: the potential of fourth-generation progestin. International journal of oncology 40(6): 1755-1762.
- Zito G, Luppi S, Giolo E, Martinelli M, Venturin I, et al. (2014) Medical treatments for endometriosis-associated pelvic pain. BioMed research international 2014: 191967.
- Chandra V, Kim JJ, Benbrook DM, Dwivedi A, Rai R (2015) Therapeutic options for management of endometrial hyperplasia. Journal of gynecologic oncology 27(1): e8.
- Rafique S, Decherney AH (2017) Medical management of endometriosis. Clinical obstetrics and gynecology 60(3): 485-496.
- Gezer A, Oral E (2015) Progestin therapy in endometriosis. Women's Health 11(5): 643-652.
- Ushijima K, Yahata H, Yoshikawa H, Konishi I, Yasugi T, et al. (2007) Multicenter phase II study of fertility-sparing treatment with medroxyprogesterone acetate for endometrial carcinoma and atypical hyperplasia in young women. Journal of clinical oncology 25(19): 2798-2803.
- Pinkerton JV (2011) Pharmacological therapy for abnormal uterine bleeding. Menopause 18(4): 459-467.
- Selak V, Farquhar CM, Prentice A, Singla AA (2001) Danazol for pelvic pain associated with endometriosis. Cochrane Database of Systematic Reviews (4): CD000068.
- Luisi S, Razzi S, Lazzeri L, Bocchi C, Severi FM, et al. (2009) Efficacy of vaginal danazol treatment in women with menorrhagia during fertile age. Fertility and sterility 92(4): 1351-1354.
- Szubert M, Suzin J, Duechler M, Szuławska A, Czyż M, et al. (2014) Evaluation of selected angiogenic and inflammatory markers in endometriosis before and after danazol treatment. Reproduction, Fertility and Development 26(3): 414-420.
- Hindman N, Eswar C, Huang K, Tong A (2020) Medical management of endometriosis: what the radiologist needs to know. Abdominal Radiology (New York) 45(6): 1866-1871.
- Vercellini P, Eskenazi B, Consonni D, Somigliana E, Parazzini F, et al. (2011) Oral contraceptives and risk of endometriosis: a systematic review and meta-analysis. Human reproduction update 17(2): 159-170.
- Sullivan H, Furniss H, Spona J, Elstein M (1999) Effect of 21-day and 24-day oral contraceptive regimens containing gestodene (60 μg) and ethinyl estradiol (15 μg) on ovarian activity. Fertility and sterility 72(1): 115-120.
- Daniels J, Middleton L, Champaneria R, Khan K, Cooper K, et al. (2012) Second generation endometrial ablation techniques for heavy menstrual bleeding: network meta-analysis. Bmj 344: e2564.
- Crosignani P, Olive D, Bergqvist A, Luciano A (2006) Advances in the management of endometriosis: an update for clinicians. Human Reproduction Update 12(2): 179-189.
- Swayzer DV, Gerriets V (2019) Leuprolide. StatPearls [Internet].
- Cook T, Sheridan WP (2000) Development of GnRH antagonists for prostate cancer: new approaches to treatment. The oncologist 5(2): 162-168.
- Lindsay SF, Luciano DE, Luciano AA (2015) Emerging therapy for endometriosis. Expert opinion on emerging drugs 20(3): 449-461.
- Alshehre SM, Duffy S, Jones G, Ledger WL, Metwally M (2020) A prospective, single-centre, single-arm, open label study of the long term use of a gonadotropin releasing hormone agonist (Triptorelin SR, 11.25 mg) in combination with Tibolone add-back therapy in the management of chronic cyclical pelvic pain. Reproductive Biology and Endocrinology 18: 28.
- Rahmawati E, Yang W CV, Lei YP, Maurya PK, Chen HW, et al. (2019) Decreased Level of Neurotrophic Factor Neuritin 1 in Women with Ovarian Endometriosis after Receiving Gonadotropin-Releasing Hormone Agonist Treatment. International journal of molecular sciences 20(18): 4352.
- Guo H, Li J, Shen X, Cong Y, Wang Y, et al. (2020) Efficacy of Different Progestins in Women With Advanced Endometriosis Undergoing Controlled Ovarian Hyperstimulation for in vitro Fertilization-A Single-Center Non-inferiority Randomized Controlled Trial. Frontiers in Endocrinology 11: 129.
- Seo JW, Lee DY, Kim SE, Yoon BK, Choi D (2019) Comparison of long-term use of combined oral contraceptive after gonadotropin-releasing hormone agonist plus add-back therapy versus dienogest to prevent recurrence of ovarian endometrioma after surgery. European Journal of Obstetrics & Gynecology and Reproductive Biology 236: 53-57.
- Akhkubekova N, Kaysinova A, Fedorov A, Efimenko N, Gaidamaka I, et al. (2018) The role of the combined balneotherapeutic treatment as the'add-back therapy'against the background of the anti-hormonal effects of the agonists of gonadotropin-releasing hormone in the women suffering from endometriosis genitalis externa. Voprosy kurortologii, fizioterapii, i lechebnoi fizicheskoi kultury 95(5): 31-39.
- Sirayapiwat P, Suwajanakorn S, Triratanachat S, Niruthisard S (2007) The effects of GnRH antagonist on the endometrium of normally menstruating women. Journal of assisted reproduction and genetics 24(12): 579-586.
- Tucci FC, Zhu YF, Struthers RS, Guo Z, Gross TD, et al. (2005) 3-[(2 R)-Amino-2-phenylethyl]-1-(2, 6-difluorobenzyl)-5-(2-fluoro-3-methoxyphenyl)-6-methylpyrimidin-2, 4-dione (NBI 42902) as a Potent and Orally Active Antagonist of the Human Gonadotropin-Releasing Hormone Receptor. Design, Synthesis, and in Vitro and in Vivo Characterization. Journal of medicinal chemistry 48(4): 1169-1178.
- Betz SF, Zhu YF, Chen C, Struthers RS (2008) Non-peptide gonadotropin-releasing hormone receptor antagonists. Journal of medicinal chemistry 51(12): 3331-3348.
- Elliott W, Chan J (2018) Elagolix (Orilissa). Internal Medicine Alert 40(18).
- Taylor HS, Dun EC, Chwalisz K (2019) Clinical evaluation of the oral gonadotropin-releasing hormone-antagonist elagolix for the management of endometriosis-associated pain. Pain management 9(5): 497-515.
- Słopień R, Męczekalski B (2016) Aromatase inhibitors in the treatment of endometriosis. Przeglad menopauzalny. Menopause review 15(1): 43-47.
- Pavone ME, Bulun SE (2012) Aromatase inhibitors for the treatment of endometriosis. Fertility and sterility 98(6): 1370-1379.
- Mori T, Ito F, Koshiba A, Kataoka H, Tanaka Y, et al. (2018) Aromatase as a target for treating endometriosis. Journal of Obstetrics and Gynaecology Research 44(9): 1673-1681.
- Hashim HA (2014) Potential role of aromatase inhibitors in the treatment of endometriosis. International journal of women's health 6: 671-680.
- Nothnick WB (2011) The emerging use of aromatase inhibitors for endometriosis treatment. Reproductive Biology and Endocrinology 9(1): 87.
- Yarmolinskaya M, Molotkov A (2019) Evaluation of the effectiveness of letrozole in the treatment of experimentally modeled endometriosis in rats. Journal of Endometriosis and Pelvic Pain Disorders 11(3): 126-131.
- Chawla S (2010) Treatment of endometriosis and chronic pelvic pain with letrozole and norethindrone acetate. Medical Journal Armed Forces India 66(3): 213-215.
- Taghavipour M, Sadoughi F, Mirzaei H, Yousefi B, Moazzami B, et al. (2020) Apoptotic functions of microRNAs in pathogenesis, diagnosis, and treatment of endometriosis. Cell & Bioscience 10(1): 1-9.
- Bjorkman S, Taylor HS (2019) MicroRNAs in endometriosis: biological function and emerging biomarker candidates. Biology of reproduction 100(5): 1135-1146.
- Marí Alexandre J, Barceló Molina M, Olcina Guillem M, García Oms J, Braza Boïls A, et al. (2018) MicroRNAs: New players in endometriosis. WJOG 5(1): 28-38.
- Rein DT, Schmidt T, Bauerschmitz G, Hampl M, Beyer IM, et al. (2010) Treatment of endometriosis with a VEGF-targeted conditionally replicative adenovirus. Fertility and sterility 93(8): 2687-2694.
- Vodolazkaia A, Yesilyurt BT, Kyama CM, Bokor A, Schols D, et al. (2016) Vascular endothelial growth factor pathway in endometriosis: genetic variants and plasma biomarkers. Fertility and sterility 105(4): 988-996.
- Rashidi BH, Sarhangi N, Aminimoghaddam S, Haghollahi F, Naji T, et al. (2019) Association of vascular endothelial growth factor (VEGF) Gene polymorphisms and expression with the risk of endometriosis: a case–control study. Molecular biology reports 46(3): 3445-3450.
- Muñoz Hernando L, Muñoz Gonzalez JL, Marqueta Marques L, Alvarez Conejo C, Tejerizo García Á, et al. (2015) Endometriosis: alternative methods of medical treatment. International journal of women's health 7: 595-603.
- Ferrero S, Ragni N, Remorgida V (2006) Antiangiogenic therapies in endometriosis. British journal of pharmacology 149(2): 133-135.
- Carmen C (2019) Angiogenesis and Antiangiogenic Treatment in Endometriosis. EC Gynaecology 8: 580-586.
- Attar R, Attar E (2015) Experimental treatments of endometriosis. Women's Health 11(5): 653-664.
- Aghajanian C, Sill MW, Darcy KM, Greer B, Mc Meekin DS, et al. (2011) Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Journal of clinical oncology 29(16): 2259-2265.
- Sherbet GV (2015) Therapeutic potential of thalidomide and its analogues in the treatment of cancer. Anticancer research 35(11): 5767-5772.
- Antônio LGL, Rosa e Silva JC, Machado DJ, Westin AT, Garcia SB, et al. (2019) Thalidomide Reduces Cell Proliferation in Endometriosis Experimentally Induced in Rats. Revista Brasileira de Ginecologia e Obstetrícia/RBGO Gynecology and Obstetrics 41(11): 668-672.
- Scarpellini F, Sbracia M, Lecchini S, Scarpellini L (2002) Anti-angiogenesis treatment with thalidomide in endometriosis: a pilot study. Fertility and Sterility 78(Suppl 1): S87.
- Habib N, Centini G, Lazzeri L, Amoruso N, El Khoury L, et al. (2020) Bowel Endometriosis: Current Perspectives on Diagnosis and Treatment. International Journal of Women's Health 12: 35-47.
- Hwang H, Chung YJ, Lee SR, Park HT, Song JY, et al. (2018) Clinical evaluation and management of endometriosis: guideline for Korean patients from Korean Society of Endometriosis 61(5): 553-564.

 Review Article
Review Article