Abstract
Background: Asymmetric dimethylarginine (ADMA), a competitive inhibitor of nitric oxide synthase (NOS), is a marker for cardiovascular disease, a relevant issue in obesity. The aims of the present study were to evaluate ADMA, Nitric Oxide (NO) concentrations and oxidative stress (PGF-2α) in obese adolescents and to assess their potential association with twenty-four hour (24-h) ambulatory blood pressure (ABP).
Methods: A group of 117 obese adolescents were recruited and compared with 83 normal-weight age and sex matched peers. In all subjects, fasting blood samples were obtained for the evaluation of ADMA, NO, insulin, blood glucose and, urine samples to measure urinary PGF-2α. In addition, a 2 h oral glucose tolerance test was performed in all obese subjects. Blood pressure was evaluated by 24-h ABP monitoring.
Results: ADMA and urinary PGF-2α (P=0.0009 and P=0.001, respectively) levels were significantly increased while NO reduced (P=0.0005) in obese adolescents compared to controls. 24-h systolic and diastolic blood pressure (SBP and DBP) as well as daytime and night-time SBP and DBP were significantly higher in obese compared to controls (all P<0.05). In a multiple stepwise linear regression analysis, the 24-h blood pressure measurements were significantly associated to ADMA concentrations. After dividing the obese group by tertiles of ADMA, SBP and DBP significantly increased across tertiles. In addition to identify predictors of ADMA concentrations, a stepwise multiple regression was performed showing that oxidative stress and BMI-SDS were the most relevant factors affecting.
Conclusions: In obese youth, ADMA levels are increased and associated with impaired blood pressure. ADMA system might represent a novel potential target for hypertension in obese youth.
Keywords:Obese adolescents; Blood pressure; Asymmetric dimethylarginine; Nitric Oxide; Oxidative Stress
Introduction
Obesity represents one of the most important risk factors
contributing to the overall burden of diseases worldwide [1,2].
Increased adiposity during childhood induces chronic metabolic and
inflammatory alterations which are directly related to endothelial
dysfunction and to the risk of cardiovascular diseases in adulthood
[3]. Endothelial dysfunction is characterized by impaired nitric
oxide (NO)-mediated vascular response and is associated with the
development of cardiovascular complications in obese subjects
[4]. NO is an endothelium derived vasoactive mediator involved in
several regulatory mechanisms within the cardiovascular system
[5]. NO is formed from the amino acid precursor L-arginine by
the enzyme NO-synthetase (NOS), whose activity is, in turn,
negatively influenced by asymmetric dimethylarginine (ADMA),
an arginine analogue. Increased ADMA concentrations have been
reported in several diseases, including chronic renal failure,
hyperhomocysteinemia, diabetes mellitus, hypercholesterolemia,
hypertriglyceridemia, hypertension, pre-eclampsia, stroke, peripheral vascular diseases, congestive heart failure and acute
coronary events [6]. NO release and/or bioavailability are also
reduced in the context of insulin resistance and type 2 diabetes [6].
In a previous study, increased plasma ADMA levels were found
in children with hypertension [7,8]. As well modifications in insulin
resistance and ADMA concentrations seem to be implicated in
changes of blood pressure moving from the pre-pubertal to the
pubertal period in obese children. However, in this previous study
no controls were available in order to characterized differences
between obese and healthy normal-weight peers. In addition,
no data on NO concentration and oxidative stress indexes were
investigated. Therefore, the present study aimed to evaluate
ADMA, NO and oxidative stress in well characterized group of only
adolescents with obesity compared to age and sex matched healthy
peers. In addition, we explored whether these markers might play
a role in the regulation of blood pressure, as assessed by 24-hour
ambulatory blood pressure (ABP) monitoring.
Methods
Study Population
We recruited 117 Caucasian obese adolescents (age (±SD): 12.1±3.1 years; range 10.1-18.0 years) who had been referred to the Obesity Clinic of the Department of Pediatrics, University of Chieti, Italy. All subjects were defined obese on the basis of body mass index (BMI) >+2 SD for age and sex, using the Italian growth charts [9]. A detailed medical and family history was obtained for all subjects. None was taking any medication known to affect body composition or blood pressure and none were affected by any medical illnesses. A complete physical examination was performed, including anthropometric parameters and staging of puberty according to Tanner criteria. Fasting blood and overnight urine samples were obtained. In obese adolescents, a standard oral glucose tolerance test (OGTT) was performed in order to evaluate glucose tolerance. As a control group, we recruited 83 normal weight (BMI between -2 and +2 SD) healthy children comparable for age (age 12.7±3.4 years; range 10.2-17.7 years), gender and pubertal stage, who had been admitted to the Department of Paediatrics of the University of Chieti for minor diseases. Control subjects were evaluated at least 8 weeks after discharge for any minor disease. The local Ethics Committee of the University of Chieti approved the study. Informed consent was obtained by parents and assent from the children.
Anthropometric Measurements
Height was measured in triplicate with a wall mounted stadiometer to the nearest 0.01 m and body weight was measured with a digital scale to the nearest 0.1 kg. BMI was expressed as kg/ m2 and the standard deviation score for BMI (BMI-SDS) for age and sex was calculated for each subjects according to Italian growth chart [9]. In all subjects, pubertal stage was defined on the basis of breast development in girls and genital development in boys, according to Tanner’s criteria.
Biochemical Procedures
Fasting plasma glucose and insulin levels were measured. Multiple aliquots of fasting blood samples were collected and stored at -80 °C for later assessment of ADMA and serum NO. All subjects performed an overnight urine collection before blood sampling. Urine samples were added with the antioxidant 4-hydroxy-tempo (Sigma Chemical Co, St Louis; Mo) and multiple aliquot samples were stored at -80°C until analysis of urinary isoprostanes (PGF- 2α).
Oral Glucose Tolerance Test and Insulin Resistance Indices
In all obese subjects, a standard 2-hour OGTT was performed according to standard procedures, as previously described [10]. HOMA-IR [(Fasting Insulin*Fasting Glucose)/22.5] was calculated as insulin resistance index. Plasma glucose level was determined by using the glucose oxidase method and plasma insulin was measured with two-site immunoenzymometric assay (AIA-PACK IRI; Tosoh, Tokyo, Japan). The limit of detection was 0.5 μU/mL with intra- and interassay coefficients of variation <7% for quality control.
ADMA
Plasma ADMA concentration was determined by a validated ELISA kit (DLD Diagnostika, Hamburg, Germany) which has previously been described and validated in detail [11]. In brief, cross-reactivity was 1.2% for symmetric dimethylarginine (SDMA) and <0.02% for L-arginine. The limit of detection was 0.05 μmol/l. The intra- and the inter-assay variation was 5.7 and 8.6 (CV %), respectively. There is a good correlation of the values measured by this ELISA and LC-tandem MS (n=29; R=0.984; P<0.0001). Reference ranges for ADMA have been established by this technique recently [12].
Nitric Oxide
Serum NO was measured in triplicate using a classical twostep assay. In detail, NO was defined by the sum of its metabolites nitrite and nitrate, defined following the conversion of nitrate to nitrite using a commercially available kit based on the Griess reaction (Total Nitric Oxide Assay kit; Assay Design, Ann Arbor, MI) [13]. The consumption of foods containing nitrates (i.e., spinach, beet, cabbage, cauliflower, beetroot, lettuce, among others) was discouraged for the 48 h preceding the measurements and evaluated by a two days dietary diary to avoid any dietary influence on the results of the NO assay.
Urinary Isoprostanes
Urinary samples were added with the antioxidant 4-hydroxytempo (Sigma Chemical Co, St Louis; Mo) and multiple aliquot samples were stored at -80°C until the analysis was performed. PGF-2α levels were evaluated in triplicate by an immuno-enzymatic method (8-iso-Prostaglandin F2α Enzyme Immunoassay kit; Assay Design) [14].
Ambulatory Blood Pressure Monitoring (ABPM)
ABPM was performed with a portable non-invasive oscillometric
recorder (SpaceLabs model 90207; Redmond, Washington,
USA). The validity of this monitor has been previously confirmed
[15,16]. The left arm was used in all children, according to the cuffs
designed, and three different cuff sizes were used (10 x 13 cm, 13 x
24 and 24 x 32 cm), selected on the basis of the arm circumference
of each child. ABP was performed during a normal day with typical
activities but children were asked to avoid vigorous exercises and to
keep their arm relaxed during each day-time inflation. Adolescents
and parents were asked to record in a diary the time they went to
bed and the time they awoke, as well as exercise periods and the
quality of the sleep. At the beginning of the evaluation period,
the accuracy and precision of the monitor was confirmed by
simultaneous measurements with a mercury sphygmomanometer.
Blood pressure readings were obtained at 20-min intervals
during daytime and at 30-min intervals during night-time. At
the end of the ABP, the monitor was downloaded to a personal
computer for the analysis of the measurements. All readings taken
during 24-h were used to calculated mean 24-h systolic blood
pressure (SBP) and diastolic blood pressure (DBP). All readings
taken during 24-h were used to calculate mean 24-h SBP, DBP
and HR, whereas mean daytime and night-time SBP, DBP and HR
were calculated based on the awake and asleep periods, calculated
from diary times [17]. Recordings were automatically rejected if
systolic blood pressure was >240 or <70mmHg or if diastolic blood
pressure was >150 or <40mmHg or if heart rate >200 or <20 beats/
min. All ambulatory blood pressure parameters were converted in
SDS using the normative values published by Wuhl, et al. [18].
Statistical Analysis
Statistical analyses were performed with SPSS (22 for Windows,
SPSS Inc., Chicago, IL). All data were expressed as mean±standard
deviation or 95%CI. For all analyses, a P-value <0.05 was considered
statistically significant. The distribution of continuous variables
was tested for skewness and kurtosis and those variables not
normally distributed were logarithmically transformed. Differences
in sex distribution were analyzed by Fisher’s exact test while
dichotomic variables were analyzed by Pearson chi-square tests.
Differences for continuous variables between obese and control
adolescents were assessed by performing the analysis of covariance
(ANCOVA), adjusting for age and sex. To identify factors affecting
blood pressure, a stepwise multiple regression was performed
using 24h SBP-SDS and DBP-SDS values as dependent variable. The
parameters included in the linear regression model as independent
variables were age, gender, BMI-SDS, HOMA-IR, ADMA and PGF-2α.
In order to further assess the effects of ADMA concentration
on ABP, obese subjects were divided according to tertiles of ADMA.
Analysis of variance with Tukey’s post-hoc testing was performed Adjustment for potential confounding factors was performed
using analysis of covariance adjusting for age, gender, BMI-SDS. In
addition to identify predictors of ADMA concentrations, a stepwise
multiple regression was performed using ADMA concentrations
as dependent variable. The parameters included in the linear
regression model as independent variables were age, gender, BMISDS,
2-hr glucose, HOMA-IR and PGF-2α. Variables of interest were
first tested singularly and then all together in a single model in
order to test the best predictors of ADMA concentrations.
Results
Obese and Control Subjects
Obese and control subjects were similar for age, sex and pubertal stage. As expected, BMI and BMI-SDS were significantly higher in obese children than in controls (both P<0.001); while no difference was documented for height (Table 1). Obese children also showed higher levels of fa sting insulin, fasting glucose and HOMA-IR values (all P< 0.001) than controls. None of the obese adolescents had impaired fasting glucose, impaired glucose tolerance (IGT) or type 2 diabetes (Table 1).
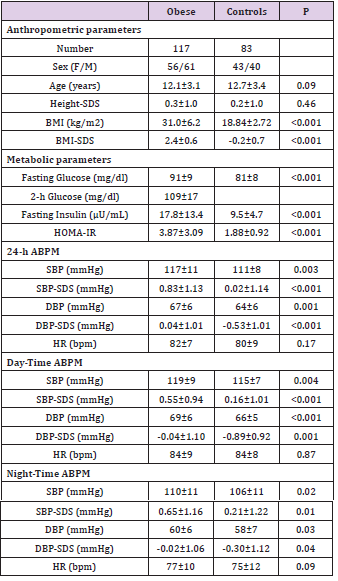
Table 1: Main anthropometric and metabolic parameters of obese and control subjects.
Data are mean±SD
M= Male; F= Female; SDS= Standard Deviation Score; BMI=
Body Mass Index; HOMA-IR= Homeostasis Model Assessment
of Insulin Resistance; SBP=Systolic Blood Pressure; DBP=
Diastolic Blood Pressure; HR= Heart Rate; ABPM= Ambulatory
Blood Pressure Measurements
ABP (Table 1)
Compared to controls, both SBP and DBP values for 24-h, dayand night-time were significantly increased in obese adolescents (all P<0.05). Similarly, both SBP-SDS and DBP-SDS values for 24-h, day- and night-time were significantly increased in obese adolescents (all P<0.05) (Table 1). No difference was documented in terms of HR. Out of 117 obese subjects, 16 adolescents presented hypertension as defined by values of systolic or diastolic or both ≥95th percentile (10 with a mean 24-h SBP value ≥95th percentile, 2 with a mean 24-h DBP value ≥95th percentile; 4 with both mean 24-h SBP and DBP values ≥95th percentile).
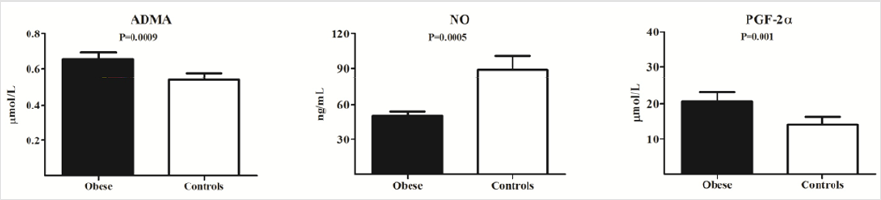
ADMA, NO and Oxidative Stress (Figure 1)
As shown in Figure 1, in obese adolescents ADMA levels were significantly higher compared to controls (0.65±0.19 vs 0.53±0.14 μmol/L, P=0.0009), whereas serum NO levels were lower (50.26±18.97 vs 87.10±43.51 mmol/L, P=0.0005). PGF-2α levels were higher in obese children compared to controls (20.62±13.42 vs 14.18±9.18 ng/ml, P=0.001) (Figure 1).
Figure 1: Asymmetric dimethylarginine (ADMA), Nitric Oxide (NO) and oxidative stress (PGF-2α) levels in Obese adolescents and controls
Factors Affecting Blood Pressure in Obese Subjects
In order to evaluate the potential influence of the most relevant variables of interest on blood pressure, a multiple stepwise regression analysis was performed using the 24h SBP-SDS and DBP-SDS as dependent variables. Of note, adjusting for age and gender, ADMA concentrations were significantly associated to both 24h SBP-SDS and DBP-SDS (P=0.001, β=0.41 and P=0.002, β=0.32) while BMI-SDS, HOMA-IR and PGF-2α were excluded by the model (all P>0.05, adjusted for age and gender).
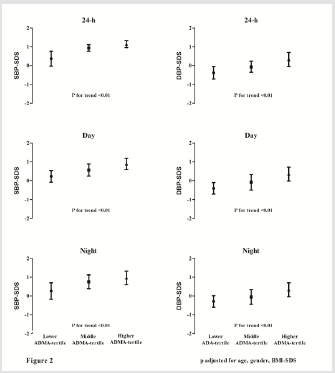
Obese Subjects Divided According to ADMA Tertile (Figure 2) and Multiple Stepwise Linear Regression Analysis (Table 2)
In order to evaluate the potential influence of ADMA concentrations on blood pressure, obese adolescents were categorized according to tertiles of ADMA (first tertile: <0.58; second tertile: 0.58 and 0.75; third tertile >0.75) (Figure 2). As shown in Figure 2, 24-h SBP-SDS and 24-h DBP-SDS progressively increased across ADMA tertiles (all P for trend P≤0.002) (Figure 2). Similarly, day- and night- both SBP-SDS and DBP-SDS, progressively increased across ADMA tertiles (all P for trend P≤0.002). As expected, all parameters used to evaluate glucose metabolism and insulin resistance as well as oxidative stress markers increased progressively according tertiles of ADMA (data not shown).
Figure 2: Distribution of 24-h-, day- and night- SBP-SDS and DBP-SDS in obese subjects divided according to ADMA tertiles (all P for trend P≤0.001).
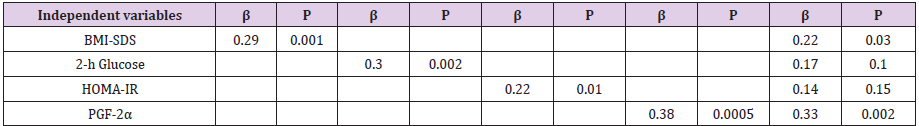
In order to evaluate factors affecting ADMA levels a multiple stepwise linear regression analysis was performed. As shown in Table 2, when the single variables of interest were included in the model, BMI-SDS, 2-h glucose, HOMA-IR and oxidative stress showed a significant and direct association with ADMA levels (Table 2). Of note, when these variables were included all together in the model, oxidative stress and BMI-SDS showed the strongest association with ADMA concentrations (Table 2).
Table 2: Multiple stepwise linear regression analysis evaluating factors influencing ADMA levels (dependent variables) in obese adolescents.
Adjusted for age and gender
BMI-SDS= Body Mass Index-Standard Deviation Score; PGF-2α= Urinary Isoprostanes
Discussion
In this study, we showed that obese adolescents had higher
ADMA and lower NO concentrations, compared to healthy matched
peers. BMI, 2-h glucose and oxidative stress were independently
determinants of ADMA levels. Notably, in this group of subjects
impaired ADMA concentrations appeared to be associated with
increased systolic and diastolic blood pressure, as assessed by
ABP, suggesting a potential role of ADMA on blood pressure
dysregulation. During the last decade, the epidemic increase in
childhood obesity has determined a shift from secondary forms
of hypertension, once predominant in this age group, to primary
hypertension, previously considered to be rare in children [19-
21]. In fact, in obese children adiposity and insulin resistance have
shown to play a major role in influencing blood pressure [22].
Release of ADMA into the plasma as well intracellular production
of ADMA inhibits NOS, reducing the bioavailability of NO [4-6].
NO is an anti-atherogenic molecule, which acts as a vasodilatant,
inhibits platelet aggregation, smooth muscle cell proliferation and
expression of adhesion molecules [4-6]. Kielstein et al. [23] showed
that systemic administration of ADMA reduces NO generation,
renal perfusion, and sodium excretion in healthy humans [23].
In a previous study in hypertensive children, plasma levels of
ADMA and of its stereoisomer, symmetric dimethylarginine, were
increased and associated with office blood pressure measurements.
In addition previous data have shown that changes in BP already
occur moving from the pre-pubertal to the pubertal period in obese
children, and modifications in insulin resistance and ADMA seem
to be implicated in this early progression in BP. However, no data
regarding NO concentrations were available and more importantly
no controls and no data on glucose tolerance status were available.
In this study we found that ADMA concentrations were
increased and associated with decreased NO values in obese
adolescents compared to healthy matched controls. Of note these
alterations in the physiological balance between ADMA/NO system
appeared to be directly related to alterations in blood pressure
regulation. In fact, a progressive increase in blood pressure values
were found across tertiles of ADMA, and this trend persisted even
after adjusting for potential confounding factors. Taken together,
our results suggest a role of ADMA as a risk factor for abnormal
blood pressure in obese adolescents, and a potential target for
future intervention strategies. Several mechanisms might explain
the finding of increased ADMA concentration in obese adolescents
[25-27]. In vitro, in vascular smooth muscle and endothelial cells
incubated in a hyperglycemic medium, as well as in streptozotocininduced
diabetic rats, hyperglycemia has been shown to reduce
dimethylarginine dimethylaminohydrolase (DDAH) activity,
resulting in increased ADMA concentration [28, 29]. In addition,
ADMA levels have been shown to be directly correlated with 2-h
glucose [30].
Similarly, an acute increase of 33%, 30% and 45% of ADMA
concentration after a 2-h OGTT has been reported in subjects
with normal glycaemia, impaired glucose tolerance and type 2
diabetes, respectively [31]. In line with these findings, we found
that 2-hour post-challenge glucose levels were associated with
ADMA levels already in a state of normal glucose tollerance. It
is thought that ADMA may also accumulate due to increased
production, as a result of redox-sensitive activation of protein
arginine N-methyltransferases (PRMTs) which form ADMA [32].
It is therefore thought that oxidative stress results in elevated
levels of ADMA that inhibit NOS activity [33]. In non-diabetic
subjects there is a significant correlation between plasma ADMA
concentrations and insulin resistance [34, 35], and improvement
in insulin sensitivity with rosiglitazone or weight loss results in
a fall in ADMA [34, 35]. In obese and elderly subjects, increased
ADMA appears to be related to decreased insulin sensitivity [30].
Interestingly in our population oxidative stress was one of the main
factors associated with ADMA levels.
The epidemic increase in childhood obesity has caused a shift
from secondary forms of hypertension, once predominant in this
age group, to primary hypertension, previously considered to be rare in children. In obese youth, systemic arterial hypertension
represents a relevant cardiovascular risk factor. We showed that
ADMA/NO system is impaired in obese youth compared with
healthy peers and seems to be directly involved in blood pressure
alteration in obese youth. Taken together, our results postulate
a role of ADMA concentrations as a risk factor for high blood
pressure in obese adolescents and a potential target for future
interventional strategies. The strength of the current study stems
from the assessment of blood pressure and the complete ADMA/
NO/oxidative stress axis in a large group of only adolescent
obese subjects compared to healthy normal-weight age, gender
and pubertal state matched peers. In addition blood pressure
was evaluated by using ABP, a simple, non-invasive method of
measuring blood pressure, which permits the observation of blood
pressure throughout day and night in a nonmedical environment
and quantification of circadian blood pressure variability and to
minimizing the effect of potential confounders, such as the white
coat effect [17, 36-38]. The major limitation of our study may be
represented by its cross-sectional design, therefore requiring
confirmation in future longitudinal studies.
In conclusion, obese youths present increased ADMA and
decreased NO levels compared with healthy matched peers,
and these markers are associated with impaired blood pressure
regulation. Adiposity and oxidative stress represent the major
factors influencing ADMA levels in this age group.
References
- Güngör NK (2014) Overweight and obesity in children and adolescents. J Clin Res Pediatr Endocrinol 6(3): 129-43.
- Lee EY, Yoon KH (2018) Epidemic obesity in children and adolescents: risk factors and prevention. Front Med 12: 658-666.
- Weihrauch-Blüher S, Schwarz P, Klusmann JH (2019) Childhood obesity: increased risk for cardiometabolic disease and cancer in adulthood. Metabolism 92: 147-152.
- Schulz E, Gori T, Munzel T (2010) Oxidative stress and endothelial dysfunction in hypertension. Hypertens Res 34(6): 665-673.
- Bruckdorfer R (2005) The basics about nitric oxide. Mol Aspects Med 26(1-2): 3-31.
- Boger RH (2006) Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med 38(2): 126-136.
- de Giorgis T, Marcovecchio ML, Giannini C, Chiavaroli V, Chiarelli F, et al. (2016) Blood pressure from childhood to adolescence in obese youths in relation to insulin resistance and asymmetric dimethylarginine. J Endocrinol Invest 39: 169-76.
- Goonasekera CD, Rees DD, Woolard P, Frend A, Shah V, et al. (1997) Nitric oxide synthase inhibitors and hypertension in children and adolescents. J Hypertens 15(8): 901-909.
- Cacciari E, Milani S, Balsamo A, Spada E, Cavallo L, et al. (2006) Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J Endocrinol Invest 29(7): 581-593.
- Jiang B, Tang G, Cao K, Wu L, Wang R (2010) Molecular mechanism for H(2)S-induced activation of K(ATP) channels. Antioxid Redox Signal 12: 1167-1178.
- Schulze F, Wesemann R, Schwedhelm E, Sydow K, Albsmeier J, et al. (2004) Determination of asymmetric dimethylarginine (ADMA) using a novel ELISA assay. Clin Chem Lab Med 42(12): 1377-1383.
- Schulze F, Maas R, Freese R, Schwedhelm E, Silberhorn E, et al. (2005) Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. Eur J Clin Invest 35(10): 622-626.
- Wennmalm A, Benthin G, Edlund A, Jungersten L, Jensen NK, et al. (1993) Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ Res 73(6): 1121-1127.
- Roberts LJ, Morrow JD (2000) Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med 28(4): 505-513.
- O'Brien E, Mee F, Atkins N, O'Malley K (1991) Accuracy of the SpaceLabs 90207 determined by the British Hypertension Society protocol. J Hypertens 9(6): 573-574.
- Reichert H, Lindinger A, Frey O, J Mortzeck, J Kiefer, et al. (1995) Ambulatory blood pressure monitoring in healthy schoolchildren. Pediatr Nephrol 9: 282-286.
- Soergel M, Kirschstein M, Busch C (1997) Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr 130: 178-184.
- Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F, et al. (2002) Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20(10): 1995-2007.
- Caprio S, Santoro N, Weiss R (2020) Childhood obesity and the associated rise in cardiometabolic complications. Nat Metab 2: 223-232.
- Wühl E (2019) Hypertension in childhood obesity. Acta Paediatr 108: 37-43.
- Muntner P, He J, Cutler JA, Wildman RP, Whelton PK (2004) Trends in blood pressure among children and adolescents. JAMA 291(17): 2107-2113.
- Marcovecchio ML, Patricelli L, Zito M, Capanna R, Ciampani M, et al. (2006) Ambulatory blood pressure monitoring in obese children: role of insulin resistance. J Hypertens 24(12): 2431-2436.
- Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, et al. (2004) Cardiovascular effects of systemic nitric oxide synthase inhibition with asymmetrical dimethylarginine in humans. Circulation 109(2): 172-177.
- White WB (2000) Ambulatory blood pressure monitoring: dippers compared with non-dippers. Blood Press Monit 5(Suppl 1): S17-23.
- Palm F, Onozato ML, Luo Z, Wilcox CS (2007) Dimethylarginine dimethylaminohydrolase (DDAH): expression, regulation, and function in the cardiovascular and renal systems. Am J Physiol Heart Circ Physiol 293(6): H3227-3245.
- Liu X, Xu X, Shang R, Chen Y (2018) Asymmetric dimethylarginine (ADMA) as an important risk factor for the increased cardiovascular diseases and heart failure in chronic kidney disease. Nitric Oxide 78: 113-120.
- Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, et al. (2003) Asymmetric dimethylarginine causes hypertension and cardiac dysfunction in humans and is actively metabolized by dimethylarginine dimethylaminohydrolase. Arterioscler Thromb Vasc Biol 23(8): 1455-1459.
- Lin KY, Ito A, Asagami T, Adimoolam S, Kimoto M, et al. (2002) Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation 106(8): 987-992.
- Lu CW, Guo Z, Feng M, Wu ZZ, He ZM, et al. (2010) Ex vivo gene transferring of human dimethylarginine dimethylaminohydrolase-2 improved endothelial dysfunction in diabetic rat aortas and high glucose-treated endothelial cells. Atherosclerosis 209(1): 66-73.
- Marliss EB, Chevalier S, Gougeon R, JA Morais, M Lamarche, et al. (2006) Elevations of plasma methylarginines in obesity and ageing are related to insulin sensitivity and rates of protein turnover. Diabetologia 49: 351-359.
- Konukoglu D, Firtina S, Serin O (2008) The relationship between plasma asymmetrical dimethyl-L-arginine and inflammation and adhesion molecule levels in subjects with normal, impaired, and diabetic glucose tolerance. Metabolism 57(1): 110-115.
- Boger RH, Sydow K, Borlak J, Thum T, Lenzen H, et al. (2000) LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ Res 87(2): 99-105.
- Blackwell S (2010) The biochemistry, measurement and current clinical significance of asymmetric dimethylarginine. Ann Clin Biochem 47(1): 17-28.
- Stuhlinger MC, Abbasi F, Chu JW, Lamendola C, Tracey L McLaughlin, et al. (2002) Relationship between insulin resistance and an endogenous nitric oxide synthase inhibitor. JAMA 287(11): 1420-1426.
- McLaughlin T, Stuhlinger M, Lamendola C, Abbasi F, Bialek J, et al. (2006) Plasma asymmetric dimethylarginine concentrations are elevated in obese insulin-resistant women and fall with weight loss. J Clin Endocrinol Metab 91(5): 1896-1900.
- Lurbe E, Torro I, Alvarez V, Nawrot T, Paya R, et al. (2005) Prevalence, persistence, and clinical significance of masked hypertension in youth. Hypertension 45(4): 493-498.
- Koch VH, Colli A, Saito MI, Furusawa EA, Ignes E, et al. (2000) Comparison between casual blood pressure and ambulatory blood pressure monitoring parameters in healthy and hypertensive adolescents. Blood Press Monit 5(5-6): 281-289.
- Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F (2002) Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20(10): 1995-2007.

 Research Article
Research Article