Abstract
Glucocorticoids (GCs) are a class of stress hormones that play a large number of biological actions in the body. The levels of GCs regulated by the intracellular enzyme 11β-hydroxysteroid dehydrogenases (11β-HSD 1 & 2) and are associated with the pathogenesis of metabolic syndrome. Here we aimed to investigate effect of Gum Arabic (GA) on GCs plasma corticosterone concentrations and its metabolic enzymes gene expression in mice. In the present study, 40 female CD-1 mice of 90 days old were randomly divided into two groups (20 of each group). Control group and GA group offered GA in the form of drink (10% w/v) for 15 weeks. GA in drinking water significantly (P<0.05) decreased food intake, body weight associated with reduction of visceral adipose tissue. GA supplementation significantly (P<0.01) decreased blood glucose, total cholesterol, and very low-density lipoprotein (VLDL) whereas increased high-density lipoprotein (HDL) concentrations compared to the control. However, supplementation of GA did not change triglycerides or very LDL. Interestingly, GA significantly (P<0.05) decreased plasma corticosterone associated with downregulation of hepatic 11β-HSD1 and glucocorticoid receptor (GR) mRNA expression compared to the control. Conversely, the treatment of GA increased hepatic 11β-HSD2 mRNA expression. In addition, the treatment of GA significantly (P<0.05) decreased muscular 11β-HSD1 compared to the control. No changes were observed in muscular GR and mineralocorticoid receptor. In conclusion, GA may enhance metabolic disorders through modification of hepatic 11β-HSDs mRNA expression which may ameliorate metabolic disorder complications. Further studies required to elucidate the molecular mechanisms of GA on GCs.
Keywords: Gum Arabic; Glucocorticoids Receptors Mice; Mineralocorticoid Receptor; 11β-Hydroxysteroid Dehydrogenases; Corticosterone
Introduction
Glucocorticoids (GCs), corticosterone in rodents and cortisol
in humans are endogenous stress hormones that exert key
physiological functions [1] on various tissues [2] including liver
[3] and skeletal muscle [4]. GCs affect almost all organs and tissues in the body [5], regulating various physiological processes
[6] including stress response [7], immune response [8], energy
metabolism [9], cell proliferation [10], skeletal muscle growth
[11], and reproduction [12]. GCs exert their action through the
glucocorticoid receptor (GR) [13], which is a transcription factor
that belongs to the superfamily of nuclear hormone receptors
[14]. GR acts via different mechanisms [15,16], one of the major
mechanisms is transcriptional regulation of its primary target
genes through genomic glucocorticoid response elements thereby
directly binding to DNA or tethering onto other DNA-binding
transcription factors [17]. Their secretion is mainly regulated by
the hypothalamic-pituitary-adrenal axis [18].
The bioactivity of GCs is regulated via the intracellular
metabolism involving 11β-hydroxysteroid dehydrogenases
(11β-HSDs) and 20-hydroxysteroid dehydrogenase (20HSD)
[19]. Elevations of GCs modify metabolic homeostasis such as
hypoglycemia [20], infections [21], trauma [22], ambiguous
temperature [23] or cold and stressful situations [24]. 11β-HSD
type 2 catalyzes the interconversion of inactive and active GCs
[25]. catalyzes the production of active corticosterone (CORT)
from 11-dehydrocorticosterone in rodents, including in liver [26]
and muscles [27]. Deregulation regulation of these enzymes has
been associated various metabolic disorders including obesity
[28], diabetes [29], hypertension [30], and cardiovascular disease
[31]. Overexpression of 11β-HSD1 in the liver [32] or muscle [33]
results in increased GCs action [34] and insulin resistance [35],
while targeted downregulation of 11β-HSD1 protect against insulin
resistance [29]. The inhibition of 11β-HSD1 decreases intracellular
GCs concentrations [36] and thus enhances insulin sensitivity both
in murine and mice [36].
On the other hand, the increased activity of 11β-HSD type 2
resulted in tissue-specific conversion of active cortisol to inactive
cortisone [37], thereby decreasing the local of active GCs levels.
Deregulation of HSD2 enzyme activity has been associated with
a number of metabolic diseases [38]. In addition, inhibition of
11βHSD type 2 has been obviously shown to induce a congenital
or acquired syndrome of mineralocorticoid exposure [27], thus
contribute to essential hypertension [30].
Gum Arabic (GA), an edible dried sticky exudate from
Acacia seyal and Acacia senegal is rich in non-viscous soluble
fibers [39]. Pharmacologically, GA has been confirmed to have
a number of therapeutic benefits including hypoglycemic [40],
hypocholesterolemic [41] immunomodulatory [42], antioxidant
[43], antiobesity [44], and many other health beneficial [45]. In
our pervious publication, we reported that GA decreased visceral
adipose tissue which was associated with downregulation of
11β-Hydroxysteroid dehydrogenase type I in the liver and muscles
of mice [46]. To our knowledge, the effect of GA on stress hormone
levels is not reported. Therefore, here we hypothesized that the
supplementation of GA in the form of drinking water may alter plasma CORT levels in mice. In addition, the alteration in plasma
CORT concentration by GA administration may associate with
changes in glucocorticoid metabolic enzymes gene and mRNA in
liver and muscle of mice. Moreover, it remains unknown whether
the changes in 11β-HSD type 1 and type 2 may be linked with
changes in glucocorticoid receptor (GR) and mineralocorticoid
receptor (MR) gene mRNA expression.
Materials and Methods
Animals and Experimental Design
Forty female albino laboratory mice (age, 90 days) old were obtained from Sudanese National Research Center and housed in 8 plastic cages (each containing 5 mice) in a room kept at 25 C with a 12-h light and dark cycle. The animals were allowed to access freely to a commercial pelleted diet for the adaptation and drinking water throughout the experiment at least for one weekday. After 7 days of adaptation, the animals were divided into two groups of 20 mice in each group. Control group and GA group. GA group was provided drinking water containing GA whereas; the control group was given tape water. These mice received 0.5% of GA aqueous solution as drinking water for 7 days to adapt GA, and then 10% solution for a further 15 weeks consecutively. The control group was remained on the same drinking water as in the acclimatization. Body weight and food consumption were recorded throughout the period of the experiment. On day 105, the mice were killed. Liver and visceral adipose tissues were dissected and weighed. The tissue and blood samples were collected and stored at-80°C.
Blood Lipid Profile Biochemistry
Plasma lipids biochemistry including total cholesterol, triglycerides, HDL, VLDL, and LDL were determined using commercially assay kits according to the manufacturers’ instructions (Nanjing Jiancheng Bioengineering Company, Nanjing, China).
Blood Glucose Measurement
Plasma samples were obtained from blood through centrifugation (2,400 rpm, 20 min, and 3.5°C) and then stored at -20 °C until samples analyzed. Blood glucose concentrations were measured using assay kits according to the manufacturers’ instructions. (Nanjing Jiancheng Bioengineering Company, Nanjing, China).
Plasma Corticosterone Measurement
After decapitation of mice, blood samples were obtained from the ruptured cervical blood vessels in heparinized tubes for corticosterone (CORT). The plasma samples were prepared after centrifugation (2,400 rpm, 20 min, 3.5°C) in a refrigerated device and frozen at –20°C until the measurement of the hormone. Plasma CORT levels were measured using radioimmunoassay method (RIA), using the CORT commercial kit according to the manufacturers’ instructions (Biochem Immuno System). All plasma samples were dosed in the same assay, to avoid inter-assay errors. The lower detection limit for CORT was 0.064 ng/mL, with a 4% intra-assay error.
Real-time PCR and Gene Expression
About 100 mg of liver and muscle were ground in liquid N2,
and a portion of about 50 to 100 mg were used for extraction of
RNA using TRIzol total RNA kit (Invitrogen, Biotechnology Co, Ltd,
Carlsbad, CA, USA) according to the manufacturer’s instruction. Two
approaches were taken to ensure that all the total RNA preparations
are free of genomic DNA contamination. Firstly, total RNAs were
treated with 10 U DNase I (Rnase Free, D2215, Takara, Japan) for 30
min at 37°C, and purified according to the manufacturer’s protocol.
Secondly, the primers for the reference gene were designed to span
an intron, so any genomic DNA contamination can be reported easily
with an extra product in the melting curves for real-time PCR. For
liver and muscle glucocorticoid metabolic genes expression, realtime
PCR was performed in Mx3000P (Stratagene, USA) according
to the previous publication [46].
Mock RT and No Template Controls were included to monitor
the possible contamination of genomic and environmental DNA at
both RT and PCR steps. The pooled sample made by mixing equal
quantity of RT products (cDNA) from all samples was used for
optimizing the PCR condition and tailoring the standard curves for
each target gene, and melting curves were performed to ensure a
single specific PCR product for each gene. The PCR products were
sequenced to validate the identity of the amplicons. Primers specific
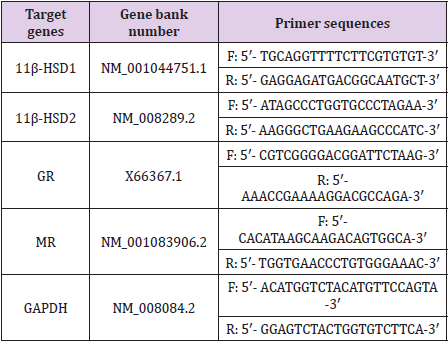
11β-HSD1, 11β-HSD2, GR and MR (Table 2) were synthesized by
Geneary (Shanghai, China). A mouse GAPDH was used as a reference
gene for normalization purposes. The method of 2−ΔΔCt was used
to analyze the real-time PCR data [47]. The mRNA abundances
were presented as the fold change relative to the average level of
the control group.
Statistical Analysis
Descriptive statistics analysis was performed to check the normality and homogeneity of variances before using parametric analyses. Body weight, food intake, organs weight, blood lipids profile, CORT, as well as the relative quantitative data of gene expression were analyzed by one-way ANOVA using SPSS 21.0 for Windows, followed by a least-significant difference (LSD) test for individual comparisons. A P-value ≤0.05 was considered significant.
Results
Body Weight, Food Intake Visceral Adipose Tissue Weight, and Liver Weight
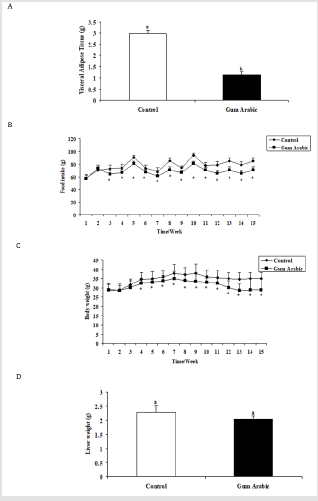
In the present study, the supplementation of GA significantly (P<0.05) decreased VAT (Figure 1A) food intake (Figure 1B), and body weight (Figure 1C) compared to the control group. No significant differences were observed in liver weight regarding the treatment of GA (Figure 1D).
Figure 1: Effect of GA treatments on liver food intake (A) body weight (B), visceral adipose tissue (C) and liver weight (D). The values are the means ± SEM, n=20/group.
Blood Glucose Lipid Profile
In this study, the treatment of GA significantly (P<0.05) decreased final blood glucose concentration compared to the control group. In addition, the administration of GA significantly (P<0.05) decreased total cholesterol (P<0.05) compared to the control group. Likewise, the supplementation of GA significantly reduced plasma LDL-c concentrations compared to the control group. On the other hand, the treatment of GA significantly (P<0.05) increased HDL-c concentrations when compared to the control group (Table 1). No changes were observed both in triglycerides or VLDL concentrations regarding GA administration.
Table 1:Effect of GA treatments on blood lipid profile and glucose concentrations. Data were expressed as means ± S.E.M. of 20 /group. Different letters in the rows indicate significantly different mean values at p<0.05
Plasma Corticosterone and Blood Glucose Concentrations
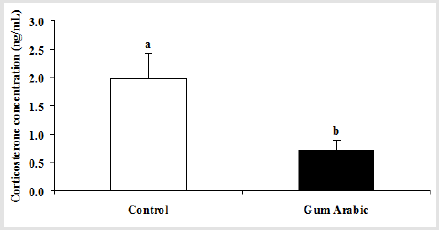
In the current study, the treatment of GA significantly (P<0.05) decreased plasma CORT concentrations when compared to the control group (Figure 2A). In addition, the administration of GA significantly (P<0.05) decreased blood glucose compared to the control group (Table 1).
Figure 2: Effect of GA treatments on plasma corticosterone concentrations (A). The values are the means ± SEM, n=20/group. Bars with different letters are significantly different at p<0.05
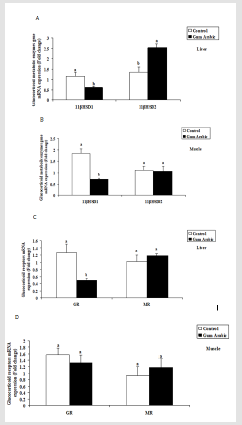
Hepatic and Muscular 11β-HSD1 and 11β-HSD2 mRNA Expression
Figure 3: Effect of GA treatments on hepatic 11βHSD1 and 11βHSD2 (A), muscle 11βHSD1 and 11βHSD2 (B), hepatic GR and MR (C) and muscle GR and MR (D) mRNA expression. The values are the means ± SEM, n=20/group. Bars with different letters are significantly different at p<0.05.
In the present study, treatment of GA significantly (P<0.05) decreased hepatic11β-HSD1 (Figure 3A) mRNA expression whereas, increased hepatic 11β-HSD2 (Figure 3A) mRNA expression compared to the control group. Similarly, the treatment of GA significantly (P<0.05) decreased muscular 11β-HSD1 (Figure 3B) mRNA expression but not 11β-HSD2 (Figure 3B) compared to the control group.
Hepatic and Muscle GR and MR mRNA Expression
In the present study, the treatment of GA significantly (P<0.05) decreased hepatic GR (Figure 3C) mRNA expression when compared to the control group. However, no significant differences observed in hepatic MR (Figure 3C) mRNA expression compared to the control group. Likewise, no significant differences were observed both in muscular GR and MR mRNA expression regarding to the treatment of GA (Figure 3D).
Discussion
There are several reports confirming the association between
dietary fibre consumption and stress relief [48-50]. Gum Arabic
(GA) is a profitable natural source of dietary fiber that reaches
85% of its weight [51]. It has a wide spectrum of health benefits
including oxidative stress [52], dyslipidemic [41], anti-obesity [53],
and anti-inflammatory [54]. In the present study, administration
of GA significantly decreased food intake associated with reduction
in body weight and blood glucose. These findings are agreed with
earlier reports that the treatment of GA associated with a reduction
in food intake [36], body weight [55] and blood glucose levels [40].
The reduction effect of GA on food intake and body weight may be
due to the fact that several studies confirmed that the dietary fiber
have bulk properties [56] and viscosity [57] therefore, it promoting
satiety [58], satiation [59, 60] and lowering body weight [61].
In addition, intake of dietary fiber associated with increases in
satiety and decreased blood glucose levels [61]. Moreover, it was
confirmed that supplementation of GA inhibited glucose absorption
in intestine through interference of membrane abundance of
sodium-glucose transporter 1 in mice [62]. Abdominal obesity is
considered the most common signs of metabolic syndrome [63].
As a result, metabolic syndrome is a fatal consequence of visceral
obesity [64]. Here we reported that supplementation of GA
decreased visceral adipose tissue (VAT) accumulation associated
with reduction in blood cholesterol, and VLDL. However, the
treatment of GA increased HDL concentrations. Our findings are
consistent with previous reports that dietary fibre decreased
adiposity [65], lowered blood cholesterol [66] and blood VLDL levels
[67]. A variety of mechanisms have been suggested to elucidate
the hypercholesterolemic effect of GA [68,69]. Some reports
suggested that the viscosity of fermentable dietary fibers in GA
contribute significantly to lipid lowering action [65,70]. In addition,
mechanism it was found that administration of GA increased fecal
bile acid excretion together with decreased medication in lipid
digestion and absorption [71].
Glucocorticoids (GCs) play a vital role in a wide array of
physiological processes in the body. The 11β-hydroxysteroid
dehydrogenases (11β-HSDs) enzyme catalyze interconversion of
intracellular GC in liver [72]. Deregulations of both 11β-HSD1 and
11β-HSD2 have been associated with several types of metabolic
disorders [73]. In the present study, the supplementation of
GA in drinking water downregulated hepatic and muscular
11β-HSD1 mRNA expression associated with decreases in plasma
corticosterone concentrations. These results are consistent
with our previous publication showed that administration of GA
decreased 11β-HSD1 mRNA expression in mice liver [46]. The
downregulation of hepatic and muscular 11β-HSD1 mRNA may
play a critical a role in the inhibition of abdominal adiposity [36]
and blood lipid profile, thus may attenuate atherosclerosis [74].
Downregulation of muscular 11β-HSD1 within the muscle may
also protect against the unfavorable effects of local inflammation
[75]. However, the mechanism through which GA downregulates
11β-HSD1 mRNA is unclear the.
Therefore, additional studies are essential to disclose
such effects. 11β-HSD2 plays a crucial role in the prevention
of inappropriate activation of the mineralocorticoid receptor
(MR) from improper activation via GCs by inactivating GCs in
mineralocorticoid target tissues [76]. In the present study, the
treatment of GA upregulated hepatic 11β-HSD2 mRNA expression.
Overexpression of hepatic 11β-HSD2 mRNA may imply the
prevention from various metabolic disorders such as atherogenesis
[36], hypertension [77] and proinflammatory changes [76].
Chronic exposure elevated levels of GCs cause metabolic disorders
such as insulin resistance [78] elevation of fasting glucose [79] and
development of type 2 diabetes mellitus [80]. Here we reported for
the first time that the administration of GA decreased GR mRNA
expression in the liver but not muscle. Downregulation of hepatic
GR mRNA could therefore decrease the development of metabolic
risk factors [81] such as obesity [82], cardiovascular diseases [83],
and diabetes [84].
Conclusion
We concluded that the supplementation of GA reduced food intake, body weight, VAT, plasma lipids profile and plasma CORT concentration which were associated with modification of hepatic 11β-HSD1, 11β-HSD2 and GR mRNA expression. Thus, GA may be useful in the treatment of metabolic disorder related diseases that induced by GCs deregulation.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The study is a collaboration projects between University of Nyala, Nyala, Sudan, Medical and Cancer Research Institute, Nyala, Sudan; University “G. d’Annunzio” of Chieti-Pescara, Italy; Islamia University, Bahawalpur, Pakistan and National Ribat University, Khartoum, Sudan, Darfur University College, University of Khartoum and Medical and Cancer Research Institute.
References
- Kuo T, C Harris, J Wang (2013) Metabolic functions of glucocorticoid receptor in skeletal muscle. Molecular and Cellular Endocrinology 380(1): 79-88.
- Patel R, Jasmine Williams-Dautovich, Carolyn L Cummins (2014) Minireview: New Molecular Mediators of Glucocorticoid Receptor Activity in Metabolic Tissues. Molecular Endocrinology 28(7): 999-1011.
- Petta I, Lien Dejager, Marlies Ballegeer, Sam Lievens, Jan Tavernier, et al. (2016) The Interactome of the Glucocorticoid Receptor and Its Influence on the Actions of Glucocorticoids in Combatting Inflammatory and Infectious Diseases. Microbiology and Molecular Biology Reviews 80(2): 495.
- Bodine SC, Furlow JD (2015) Glucocorticoids and Skeletal Muscle. Adv Exp Med Biol 872: 145-176.
- Oakley RH, Cidlowski JA (2013) The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. The Journal of allergy and clinical immunology 132(5): 1033-1044.
- Ramamoorthy S, Cidlowski JA (2016) Corticosteroids: Mechanisms of Action in Health and Disease. Rheumatic diseases clinics of North America 42(1): 15-31.
- Finsterwald C, Alberini CM (2014) Stress and glucocorticoid receptor-dependent mechanisms in long-term memory: from adaptive responses to psychopathologies. Neurobiology of learning and memory 112: 17-29.
- Liberman AC, Budzinski ML, Sokn C, Gobbini RP, Steininger A et al. (2018) Regulatory and Mechanistic Actions of Glucocorticoids on T and Inflammatory Cells. Frontiers in endocrinology 9: 235-235.
- Bordag N, Klie S, Jürchott K, Vierheller J, Schiewe H, et al. (2015) Glucocorticoid (dexamethasone)-induced metabolome changes in healthy males suggest prediction of response and side effects. Scientific Reports 5: 15954.
- Whirledge S, Dixon D, Cidlowski JA (2012) Glucocorticoids regulate gene expression and repress cellular proliferation in human uterine leiomyoma cells. Hormones & cancer 3(3): 79-92.
- Braun TP, Marks DL (2015) The regulation of muscle mass by endogenous glucocorticoids. Frontiers in Physiology 6(12).
- Whirledge S, Cidlowski JA (2017) Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends in endocrinology and metabolism: TEM 28(6): 399-415.
- Desmet SJ, De Bosscher K (2017) Glucocorticoid receptors: finding the middle ground. J Clin Invest 127(4): 1136-1145.
- Mazaira GI, Zgajnar NR, Lotufo CM, Becerra CD, Sivils J, et al. (2018) The Nuclear Receptor Field: A Historical Overview and Future Challenges. Nucl Receptor Res 5.
- Scheschowitsch K, Leite JA, Assreuy J (2017) New Insights in Glucocorticoid Receptor Signaling-More Than Just a Ligand-Binding Receptor. Frontiers in endocrinology 8: 16-16.
- Cain DW, Cidlowski JA (2015) Specificity and sensitivity of glucocorticoid signaling in health and disease. Best practice & research. Clinical endocrinology & metabolism 29(4): 545-556.
- Hudson WH, Ian Mitchelle S de Vera, Jerome C Nwachukwu, Emily R Weikum, Austin G Herbst, et al. (2018) Cryptic glucocorticoid receptor-binding sites pervade genomic NF-κB response elements. Nature communications 9(1): 1337-1337.
- Burford NG, Natalia A Webster, Diana Cruz-Topete (2017) Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int J Mol Sci 18(10).
- Wyrwoll CS, M Holmes, J Secki (2011) 11β-hydroxysteroid dehydrogenases and the brain: from zero to hero, a decade of progress. Frontiers in neuroendocrinology 32(3): 265-286.
- Kaur J (2014) A comprehensive review on metabolic syndrome. Cardiology research and practice.
- Graziadio C, Hasenmajer V, Venneri MA, Gianfrilli D, Isidori AM, et al. (2018) Glycometabolic Alterations in Secondary Adrenal Insufficiency: Does Replacement Therapy Play a Role? Frontiers in endocrinology 9: 434-434.
- Alarcon JD, Rubiano A, Okonkwo DO, Alarcon J, Martinez‐Zapata MJ, et al. (2017) Elevation of the head during intensive care management in people with severe traumatic brain injury. The Cochrane database of systematic reviews 12(12): CD009986-CD009986.
- Reul JMHM, Collins A, Richard S Saliba, Karen R Mifsud, Sylvia D Carter, et al. (2015) Glucocorticoids, epigenetic control and stress resilience. Neurobiology of Stress 1: 44-59.
- Mifsud KR, Reul JMHM (2018) Mineralocorticoid and glucocorticoid receptor-mediated control of genomic responses to stress in the brain. Stress 21(5): 389-402.
- Patel H, Dhangar K, Sonawane Y, Surana S, Karpoormath R, et al. (2018) In search of selective 11β-HSD type 1 inhibitors without nephrotoxicity: An approach to resolve the metabolic syndrome by virtual based screening. Arabian Journal of Chemistry 11(2): 221-232.
- Seckl JR, Walker BR (2001) Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology 142(4): 1371-1376.
- Chapman K, M Holmes, J Seckl (2013) 11β-hydroxysteroid dehydrogenases: intracellular gate-keepers of tissue glucocorticoid action. Physiological reviews 93(3): 1139-1206.
- Dube S, Norby BJ, Pattan V, Carter RE, Basu A, et al. (2015) 11β-hydroxysteroid dehydrogenase types 1 and 2 activity in subcutaneous adipose tissue in humans: implications in obesity and diabetes. The Journal of clinical endocrinology and metabolism 100(1): E70-E76.
- Shukla R, Basu AK, Mandal B, Mukhopadhyay P, Maity A, et al. (2019) 11β Hydroxysteroid dehydrogenase - 1 activity in type 2 diabetes mellitus: a comparative study. BMC endocrine disorders 19(1): 15.
- Ferrari P (2010) The role of 11β-hydroxysteroid dehydrogenase type 2 in human hypertension. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1802(12): 1178-1187.
- Gray GA, Christopher I White, Raphael F P Castellan, Sara J McSweeney, Karen E Chapman (2016) Getting to the heart of intracellular glucocorticoid regeneration: 11β-HSD1 in the myocardium. Journal of molecular endocrinology 58(1): R1-R13.
- Dube S, Michael Q Slama, Ananda Basu, Robert A Rizza, Rita Basu (2015) Glucocorticoid Excess Increases Hepatic 11β-HSD-1 Activity in Humans: Implications in Steroid-Induced Diabetes. The Journal of clinical endocrinology and metabolism 100(11): 4155-4162.
- Morgan SA, Sherlock M, Gathercole L, Lavery G, Lenaghan C, et al. (2009) 11beta-hydroxysteroid dehydrogenase type 1 regulates glucocorticoid-induced insulin resistance in skeletal muscle. Diabetes 58(11): 2506-2515.
- Morgan SA, Emma L McCabe, Laura L Gathercole, Zaki K Hassan Smith, Dean P Larner, et al. (2014) 11β-HSD1 is the major regulator of the tissue-specific effects of circulating glucocorticoid excess. Proceedings of the National Academy of Sciences of the United States of America 111(24): E2482-E2491.
- Schnackenberg CG (2013) Chronic inhibition of 11 β -hydroxysteroid dehydrogenase type 1 activity decreases hypertension, insulin resistance, and hypertriglyceridemia in metabolic syndrome. BioMed research international pp. 427640-427640.
- Chang LL, Wan-Song Alfred Wun, Paulus S Wang (2018) An inhibitor of 11-β hydroxysteroid dehydrogenase type 1 (PF915275) alleviates nonylphenol-induced hyperadrenalism and adiposity in rat and human cells. BMC pharmacology & toxicology 19(1): 45-45.
- Krozowski Z, KXZ Li, K Koyama, RE Smith, VR Obeyesekere, et al. (1999) The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J Steroid Biochem Mol Biol 69(1-6): 391-401.
- Iozzo P, Holmes MC, Schmidt M, Cirulli F, Guzzardi MA, et al. (2014) Developmental ORIgins of Healthy and Unhealthy AgeiNg: the role of maternal obesity-introduction to DORIAN. Obesity facts 7(2): 130-151.
- Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5(4): 1417-35.
- Roozbeh N, Darvish L, Abdi F (2017) Hypoglycemic effects of Acacia nilotica in type II diabetes: a research proposal. BMC research notes 10(1): 331-331.
- Mohamed RE, Rima E Mohamed, Mohammed O Gadour (2015) The lowering effect of Gum Arabic on hyperlipidemia in Sudanese patients. Front Physiol 6: 160.
- Sekhon BK, Roubin RH, Yiming Li, Parimala B Devi, Nammi S, et al. (2016) Evaluation of Selected Immunomodulatory Glycoproteins as an Adjunct to Cancer Immunotherapy. PloS one 11(1): e0146881-e0146881.
- Mirghani M, Ahmed AM Elnour, Kabbashi NA, Md Z Alam, Musa KH, et al. (2018) Determination of antioxidant activity of gum Arabic: An exudation from two different locations. Science Asia 44(3): 179.
- Ushida K (2012) Gum Arabic and its Anti-obese Effect. In Gum Arabic, pp. 285-290.
- Ahmed AA (2018) 16 - Health Benefits of Gum Arabic and Medical Use. In Gum Arabic (Mariod, A.A. ed), pp. 183-210.
- Ahmed AA, Hassan H Musa, Jaafar S Fedail, Amal Z Sifaldin, Taha H Musa (2015) Gum arabic decreased visceral adipose tissue associated with downregulation of 11β-hydroxysteroid dehydrogenase type I in liver and muscle of mice. Bioactive Carbohydrates and Dietary Fibre 6(1): 31-36.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402-408.
- Al-Dujaili AE, S Ashmore (2019) A Short Study Exploring the Effect of the Glycaemic Index of the Diet on Energy intake and Salivary Steroid Hormones. Nutrients 11(2).
- Rushen J, S Ashmore (2016) Effects of an oat-based high-fibre diet on insulin, glucose, cortisol and free fatty acid concentrations in gilts. Animal Science 69(2): 395-401.
- Laugero KD, Luis M Falcon, Katherine L Tucker (2011) Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite 56(1): 194-204.
- Mariod AA (2018) 20 - Gum Arabic Dietary Fiber. In Gum Arabic (Mariod, A.A. ed), pp. 237-243.
- Ahmed AA, Jaafar S Fedail, Hassan H Musa, Asghar Ali Kamboh, Amal Z Sifaldin, et al. (2015) Gum Arabic extracts protect against hepatic oxidative stress in alloxan induced diabetes in rats. Pathophysiology 22(4): 189-194.
- Ahmed AA, Hassan H Musa, Jafaar S Fedail, Amal Z Sifaldin, Taha H Musa (2016) Gum arabic suppressed diet-induced obesity by alteration the expression of mRNA levels of genes involved in lipid metabolism in mouse liver. Bioactive Carbohydrates and Dietary Fibre 7(1): 15-20.
- Kamal E, Gadir Kaddam LA, Maha Dahawi, Montaser Osman, Salih MA, et al. (2018) Gum Arabic Fibers Decreased Inflammatory Markers and Disease Severity Score among Rheumatoid Arthritis Patients, Phase II Trial. International Journal of Rheumatology.
- Babiker R, Tarig H Merghani, Khalifa Elmusharaf, Rehab M Badi, Florian Lang, et al. (2012) Effects of Gum Arabic ingestion on body mass index and body fat percentage in healthy adult females: two-arm randomized, placebo controlled, double-blind trial. Nutr J 11: 111.
- Tan C, Wei H, Zhao X, Xu C, Peng J (2017) Effects of dietary fibers with high water-binding capacity and swelling capacity on gastrointestinal functions, food intake and body weight in male rats. Food Nutr Res 61(1): 1308118.
- Dikeman CL, Fahey GC (2006) Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr 46 (8): 649-663.
- Warrilow A, Duane Mellor, Andrew McKune, Kate Pumpa (2019) Dietary fat, fibre, satiation, and satiety-a systematic review of acute studies. Eur J Clin Nutr 73(3): 333-344.
- Salleh SN, Hamizi Fairus AA, Zahary MN, Raj NB, Mhd Jalil AM (2019) Unravelling the Effects of Soluble Dietary Fibre Supplementation on Energy Intake and Perceived Satiety in Healthy Adults: Evidence from Systematic Review and Meta-Analysis of Randomised-Controlled Trials. Foods 8(1): 15.
- Warrilow A, Duane Mellor, Andrew McKune, Kate Pumpa (2019) Dietary fat, fibre, satiation, and satiety—a systematic review of acute studies. European Journal of Clinical Nutrition 73(3): 333-344.
- Thompson SV, Bridget A Hannon, Ruopeng An, Hannah D Holscher (2017) Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 106(6): 1514-1528.
- Nasir O, Artunc F, Wang K, Rexhepaj R, Föller M, et al. (2010) Downregulation of mouse intestinal Na(+)-coupled glucose transporter SGLT1 by gum arabic (Acacia Senegal). Cell Physiol Biochem 25(2-3): 203-10.
- Paley CA, Johnson MI (2018) Abdominal obesity and metabolic syndrome: exercise as medicine? BMC sports science, medicine & rehabilitation 10(1): 7.
- Lopes HF, Correa-Giannella ML, Consolim-Colombo FM, Egan BM (2016) Visceral adiposity syndrome. Diabetology & metabolic syndrome 8: 40.
- Brockman DA, Xiaoli Chen, Daniel D Gallaher (2014) High-viscosity dietary fibers reduce adiposity and decrease hepatic steatosis in rats fed a high-fat diet. J Nutr 144(9): 1415-1422.
- Narayan S, Lakshmipriya N, Vaidya R, Ramya Bai M, Sudha V, et al. (2014) Association of dietary fiber intake with serum total cholesterol and low-density lipoprotein cholesterol levels in Urban Asian-Indian adults with type 2 diabetes. Indian journal of endocrinology and metabolism 18(5): 624-630.
- Hannon BA, Sharon V Thompson, Caitlyn G Edwards, Sarah K Skinner, Grace M Niemiro, et al. (2018) Dietary Fiber Is Independently Related to Blood Triglycerides Among Adults with Overweight and Obesity. Current Developments in Nutrition 3(2).
- Kelley JJ, Tsai AC (1978) Effect of pectin, gum arabic and agar on cholesterol absorption, synthesis, and turnover in rats. J Nutr 108(4): 630-639.
- Moundras C, Behr SR, Demigne C, Mazur A, C Remesy (1994) Fermentable polysaccharides that enhance fecal bile acid excretion lower plasma cholesterol and apolipoprotein E-rich HDL in rats. J Nutr 124(11): 2179-88.
- Schroeder N, Len F Marquart, Gallaher DD (2013) The role of viscosity and fermentability of dietary fibers on satiety- and adiposity-related hormones in rats. Nutrients 5(6): 2093-2113.
- Eastwood MA (1992) The Physiological Effect of Dietary Fiber: An Update. Annual Review of Nutrition 12: 19-35.
- Stimson RH, Ruth Andrew, Norma C McAvoy, Dhiraj Tripathi, Peter C Hayes, et al. (2011) Increased Whole-Body and Sustained Liver Cortisol Regeneration by 11β-Hydroxysteroid Dehydrogenase Type 1 in Obese Men with Type 2 Diabetes Provides a Target for Enzyme Inhibition. Diabetes 60(3): 720-725.
- Bailey MA (2017) 11β-Hydroxysteroid Dehydrogenases and Hypertension in the Metabolic Syndrome. Current hypertension reports 19(12): 10-100.
- Garcia RA, Debra J Search, John A Lupisella, Jacek Ostrowski, Bo Guan, et al. (2013) 11β-Hydroxysteroid Dehydrogenase Type 1 Gene Knockout Attenuates Atherosclerosis and In Vivo Foam Cell Formation in Hyperlipidemic apoE−/− Mice. PLOS ONE 8(2): e53192.
- Hardy RS, Craig L Doig, Zahrah Hussain, Mary O'Leary, Stuart A Morgan, et al. (2016) 11β-Hydroxysteroid dehydrogenase type 1 within muscle protects against the adverse effects of local inflammation. The Journal of pathology 240(4): 472-483.
- Hadoke PWF, Kipari T, Seckl JR, Chapman KE (2013) Modulation of 11β-hydroxysteroid dehydrogenase as a strategy to reduce vascular inflammation. Current atherosclerosis reports 15(5): 320-320.
- Tapia-Castillo A, Cristian A Carvajal, Fidel Allende, Carmen Campino, Carlos E Fardella (2017) Hypertensive Patients That Respond to Aldosterone Antagonists May Have a Nonclassical 11β-HSD2 Deficiency. American Journal of Hypertension 30(8): e6-e6.
- Zhou PZ, Zhu YM, Zou GH, Sun YX, Xiu XL, et al. (2016) Relationship Between Glucocorticoids and Insulin Resistance in Healthy Individuals. Medical science monitor. International Medical Journal of Experimental and Clinical Research 22: 1887-1894.
- Kamba A, Daimon M, Murakami H, Otaka H, Matsuki K, et al. (2016) Association between Higher Serum Cortisol Levels and Decreased Insulin Secretion in a General Population. PloS one 11(11): e0166077-e0166077.
- Suh S, Park MK (2017) Glucocorticoid-Induced Diabetes Mellitus: An Important but Overlooked Problem. Endocrinology and metabolism (Seoul, Korea) 32(2): 180-189.
- Sekgala MD, Zandile J Mchiza, Whadi-ah Parker, Kotsedi D Monyeki (2018) Dietary Fiber Intake and Metabolic Syndrome Risk Factors among Young South African Adults. Nutrients 10(4): 504.
- Hadrévi J, Sogaard K, Christensen JR (2017) Dietary Fiber Intake among Normal-Weight and Overweight Female Health Care Workers: An Exploratory Nested Case-Control Study within FINALE-Health. Journal of nutrition and metabolism pp. 1096015-1096015.
- Anderson JW, Pat Baird, Richard H Davis, Stefanie Ferreri, Mary Knudtson, et al. (2009) Health benefits of dietary fiber. Nutr Rev 67(4): 188-205.
- Weickert MO, Pfeiffer AFH (2018) Impact of Dietary Fiber Consumption on Insulin Resistance and the Prevention of Type 2 Diabetes. J Nutr 148(1): 7-12.

 Research Article
Research Article