Abstract
Background: Biosafety and biosecurity compliant laboratories play a crucial role in the successful implementation of the International Health Regulation (IHR) 2005 in a country. Biosafety implementation in laboratories require legislative framework (laws, guidelines, and standards), a roadmap for biosafety program implementation, biosafety training, monitoring and supervision of biosafety practices, and a biosafety certification program. Here we present the initiatives taken by the Ministry of Health (MoH) in Vietnam to strengthen biosafety practices in clinical and research laboratories from 2007 to 2017.
Methods: We collected data from the MoH and Department of Health (DoH) database on number of laboratories. Biosafety and biosecurity related laws, decrees, and circulars issued by the MoH or DoH were collected from the website of the General Department of Preventive Medicine (GDPM) (http://www.vncdc.gov.vn/he-thong-van-ban). We collected biosafety capacity building initiatives data (e.g. biosafety training and biosafety accreditation) from the GDPM and its central and regional institutions and DoH database.
Results: There is 2420 biosafety level (BSL) 1 and 2 laboratories and 3 BSL3 laboratories in Vietnam including 657 in the North, 500 in the Central and 1263 in the South region. From 2007 to 2017, Government of Vietnam including MoH have issued or revised 10 biosafety and biosecurity legislation including one law, two decree, and seven circulars. These legislations provided guidelines for the implementation of biosafety and biosecurity practices. From 2007 to 2017, 1490 (61.75%; 1490/2420) BSL1 and 2 laboratories and all BSL3 laboratories were certified for biosafety compliance. During this period, 13,012 laboratory staff were trained on biosafety and biosecurity including 4622 staff in the North, 5695 staff in South and 2695 in the central region of Vietnam.
Keywords:Biosafety; Biosecurity; Vietnam; Laboratory
Background
Outbreaks of disease in humans and animals and their crossspecies transmission pose a significant risk to the health of the population, poultry, fisheries and livestock, and could influence business, economy, tourism, and reputation of a country [1]. To prevent and protect population from bio-terror (from the act of bioterrorism) or bio-error (accidental or incidental release of pathogens) three Internationally mandated regulations including the International Health Regulations (IHR) 2005 (WHO 2005); United Nations Security Council Resolution 1540 (United Nations Security Council 2004); and the Biological Weapons Convention (Biological and Toxin Weapons Convention 1975) are being implemented globally [2]. Under IHR 2005, states are legally bound to improve the core capabilities “to prevent, protect against, control and provide a public health response to the international spread of disease in ways that are commensurate with and restricted to public health risks, and which avoid unnecessary interference with international traffic and trade” [2,3].
IHR 2005 requires countries to strengthen eight-core capacities (national legislation, coordination and communication, surveillance, response, preparedness, risk communication, human resource capacity and laboratory), on four hazards (zoonotic events, chemical events, food safety, radiation emergencies), at the points of entry to a country. Strengthening laboratory core capabilities to comply with IHR 2005 would require i) availability of laws, regulations and guidelines, ii) designation of responsible entity for biosafety and biosecurity, iii) biorisk assessment, iv) availability and accessibility of biosafety guidelines, v) biosafety training, vi) national classification of microorganism by risk group, and vii) laboratory biosafety certification program [4,2].
Laboratories play a vital role in the successful implementation of IHR 2005 in all three key areas including “prevent”, “detect” and “respond”. This includes i) diagnosis of endemic or exotic infectious diseases in humans or animals, ii) detection of contamination of foods with infectious pathogens or toxins, and iii) disease surveillance with detection and reporting of infectious diseases of public health interest. Besides this, laboratories are involved in receiving, transport, analyze, storage or disposal of pathogens and biological samples. Therefore, biosafety and biosecurity measures in laboratories are crucial for containing the spread of diseases, to prevent Laboratory Acquired Infections (LAIs), the transmission of infections to the community, reduce contamination of the environment, prevent the risk of access/possession of pathogens by unauthorized individuals, and the threats of bioterrorism [5].
Biosafety and biosecurity implementation in public and private laboratories in the Asia Pacific region including Vietnam are not optimum [6]. Lack of appropriate legislations, policies, regulations, the absence of appropriate strategies, limitations of resources, lack of training opportunities, and limited international assistance are a few reasons to mention [7]. As a signatory of IHR 2005, Ministry of Health (MoH), Vietnam along with other relevant ministries have taken steps to enhance the biosafety and biosecurity in laboratories in Vietnam. Here, we present the initiatives, the key successes, and the major challenges in strengthening the biosafety and biosecurity in clinical and research laboratories in Vietnam.
Activities
Administrative and Technical Management of Laboratories
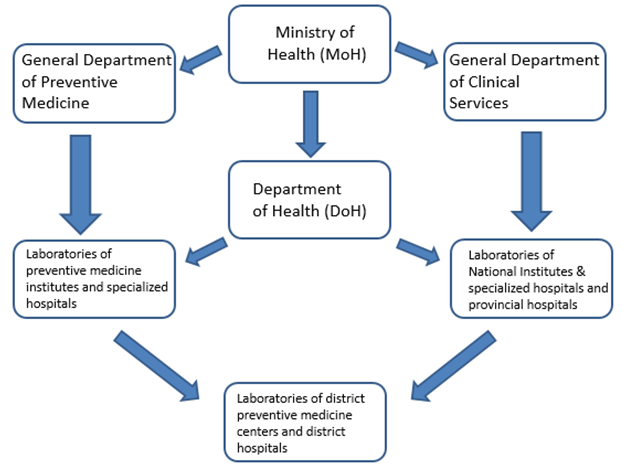
Figure 1: The laboratory system is classified as national, provincial and city, and district levels laboratories.
The health care system in Vietnam is delivered through the “General Department of Clinical Services (GDCS)” and “General Department of Preventive Medicine (GDPM)” under the MoH. GDPM oversees the laboratories of central and regional preventive medicine institutes and specialized hospitals (e.g., National Hospital for Tropical Diseases, Hanoi and Hospital for Tropical disease in Ho Chi Minh City). GDMP is also responsible for biosafety of the laboratories handling infectious disease samples for diagnosis and research. GDCS oversees the national institute and specialized hospital’s clinical laboratories and coordinates the clinical laboratory services of the provincial hospitals through DoH. Preventive medicine laboratory services at district hospitals and commune health posts are under the preventive medicine department of DoH. The laboratory system is classified as national, provincial and city, and district levels laboratories (Figure 1). The higher levels of laboratories are responsible for guiding, training, and monitoring the lower level laboratories, e.g. provincial hospital laboratories oversee and support district hospital laboratories. MoH maintains a database of laboratories and DoH maintains a database for laboratory safety certification. The central and provincial preventive medicine institutes maintain a database of laboratory safety training.
Biosafety and Biosecurity Legislation
The Vietnamese legislation hierarchy includes laws (ND), decree (QD), circulars (TT), and technical requirements (QCVN). Government/parliament issues laws and decrees and MoH, technical expert committee issues circulars and technical requirements. Experts from different disciplines form specialized committees and/or task forces to develop and review the decrees, decisions, and circulars.
Legislations on Biosafety Curriculum and Training
Biosafety and biosecurity have been given priority in health regulations and mandatory biosafety training was implemented for all staff working in medical laboratories. Through circular No: 22/2013/TT-BYT a comprehensive biosafety training curriculum and training guidelines were established (S File 1) in 2013. This circular provides guidelines for biosafety training requirements, training organization, and management of biosafety training programs. The training program targeted to train more than 10,000 health professionals by 2020.
Biosafety Certification Program
Laboratory biosafety certification program was implemented through circular 29/2012/TT-BYT. This includes the formation of a central/provincial body for implementation and management of biosafety certification program, identification and training of auditors, and development of a checklist for biosafety certification (S File 2, S File 3). DoH in each city/province is responsible for certification of BSL1 and 2 laboratories, and MoH is responsible for certification of BSL3 laboratories. For BSL 1 and 2 laboratories, the certification was valid for three years and for BSL3 laboratories, the certification was valid for one years. A new system for selfassessment of biosafety compliance was introduced in July 2016, through “Decree No 103/216/ND-CP. This allows laboratories to do a self-assessment of compliance with the biosafety regulations and report to the DoH.
Response to Emerging Disease Outbreak
To respond the emerging infectious disease outbreaks (e.g. Severe Acute Respiratory Syndrome (SARS), human infection with influenza A (H5N1) virus, Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and influenza A (H7N9) virus,, Ebola and Zika virus) MoH/DoH and other ministries develop task force/ working group for specific situation. The task force/working group develops the necessary guideline, identifies key institutes to handle the patients, conduct diagnostic tests, rollout training programs for first responders (health care workers, nurses, laboratory staff and doctors), and provide recommendations for improving patient management and diagnostic capacity.
Global Health Security (GHS) Preparedness
To assess the national preparedness for a coordinated response to any infectious disease outbreak, MoH/DoH and GDPM conducts GHS drill exercises [8]. The exercise focused on establishing an emergency operations center (EOC) at the GDPM, assessing the nationwide laboratory system, diagnostic capacity for priority pathogens (i.e., H5N1, MARS CoVI), and communication and coordination during an epidemic outbreak. Similar EOC are established during outbreaks and epidemics.
National and International Collaboration
MoH, Vietnam facilitates bilateral and multilateral collaboration with different countries and international agencies for strengthening biosafety and biosecurity capacity. These collaborations result in infrastructure improvement, human resource capacity building, training capacity building and implementation of biosafety and biosecurity monitoring systems.
Achievements
Management of Laboratories
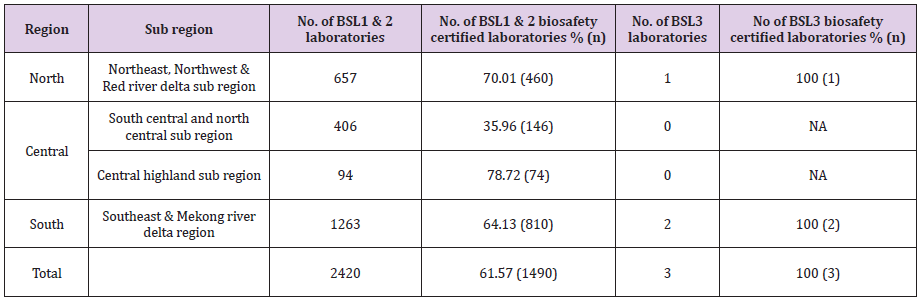
There are 2,420 BSL1 and BSL2 clinical and research laboratories in Vietnam. Of these, 657 laboratories are located in the North, 500 in the Central, and 1263 in the South (Table 1) region. Additionally, there are 73 preventive medicine diagnostic and reference laboratories and 3 specialized institute on Malariology, parasitology and Entamology in Vietnam. This includes four national/regional public health laboratories ((National Institute of Hygiene and Epidemiology (NIHE), Pasteur Institute Ho Chi Minh City (PI-HCMC), Nha Trang Pasteur Institute (PINT), Central Highlands Institute of Hygiene and Epidemiology). There are six intermediate level public health laboratories, including two public health laboratories (National Hospital for Tropical Diseases in Ha Noi, Hospital of Tropical Diseases in Ho Chi Minh City), two nongovernmental research laboratories (Oxford University Clinical Research Unit (OUCRU) laboratories in HCMC and Ha Noi), and two paediatric hospital laboratories (National Pediatric Hospital in Hanoi and Children Hospital Number 2 in HCMC). Additionally, there are 63 district public health laboratories in Vietnam.
Table 1: Number of laboratories and number of biosafety certified laboratories in each regions of Vietnam.
NA: not applicable
Legislation on Laboratory Biosafety and Biosecurity
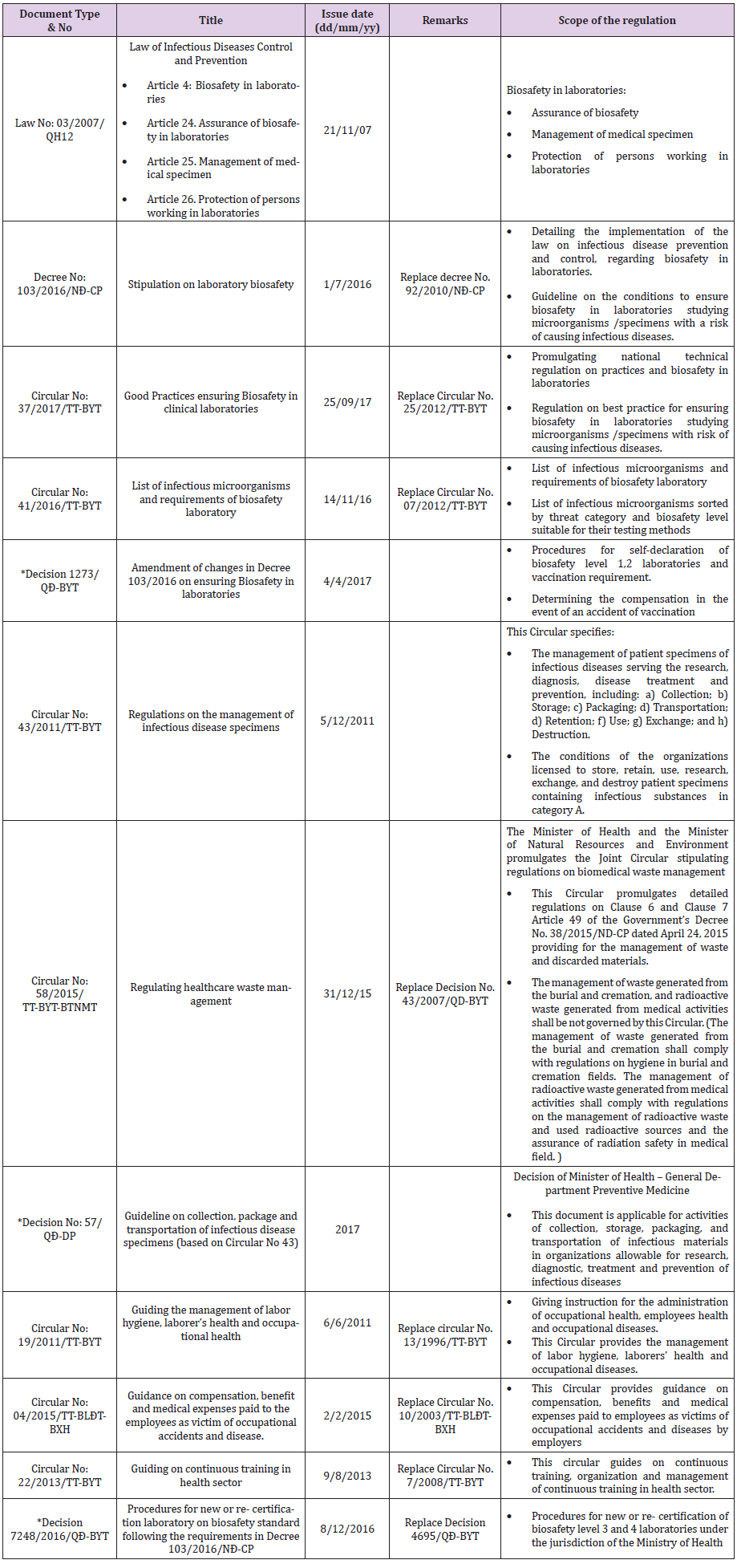
From 2010 to 2017, MoH/DoH issued 10 legislations (one law, two decree, and seven circulars) to enhance biosafety and biosecurity (Table 2) practices. Table 2 presents a summary of the legislations (number, title, issue date, and scope of the legislation). This includes legislation on the establishment, operation, and certification of the laboratories in the public and private sectors. The “Law of communicable disease control and prevention (03/2007/ QH12)” provides i) framework for biosafety in laboratories, ii) management of medical specimen, and iii) protection of staff working in the laboratories. The decree 103/2016/NĐ-CP “Stipulation on laboratory biosafety” provides a guideline for implementation of the law on communicable disease prevention and control, biosafety in the laboratories. The minimum requirement for diagnosis and handling of microorganisms was implemented through circular 41/2016/TT-BYT. Biological specimen management guideline was implemented through circular 43/2011/TT-BYT. In 2017, circular 37/2017/TT-BYT was implemented to describe the national technical regulation of laboratory facility, equipment, personnel, and standard laboratory practice.
Table 2: List of key law, decree, decisions, and circulars issued by MoH, Vietnam related to biosafety and biosecurity in clinical and research laboratories in Vietnam
*; “Decision” are not legislative document. Document source (URL): on the website of General department of preventive medicine – Regulations system (“Hệ thống văn bản”)http://vncdc.gov.vn/vi/he-thong-van-ban
Standards for medical waste and medical wastewater management were implemented through circular 58/2015/TTLTBYT- BTNMT and QCVN 28:2010/BTNMT respectively (Table 2). Guidance on periodic health checkups of laboratory staff was implemented through circular 19/2011/TT-BYT. Along with this, circular 04/2015/TT-BLĐTBXH “Guidance on compensation, benefits and medical expenses paid to employees as a victim of occupational accident and diseases” was implemented to support laboratory staff in the event of LAIs. Besides this, through Decision 35/2005/QĐ-BYT standards for Microbiological, Biochemistry, Hematology, Blood bank laboratories, was implemented. Standards for Preventive Medicine laboratories were implemented through Decision 4696/QĐ-BYT.
Through these legislations, a minimum criteria for a laboratory (infrastructure, instruments, and laboratory practices) were established and laboratories are classified into four categories (BSL1-4). A pathogen classification list was developed, and all pathogens were divided into four groups (CL1-4). The classification considered the endemic nature of certain disease in Vietnam including Dengue, TB, salmonella, etc. and a risk-based laboratory containment strategy was implemented. Procedures for specimen collection, primary processing and transport were established. Comprehensive waste management including segregation at the origin, color-coded waste collection containers (green for general waste, yellow for clinical and infectious waste and black for chemical waste) and waste autoclave procedure are implemented. Evaluation of microbial contamination of wastewater, wastewater treatment before releasing to the environment is implemented.
Biosafety Curriculum and Training
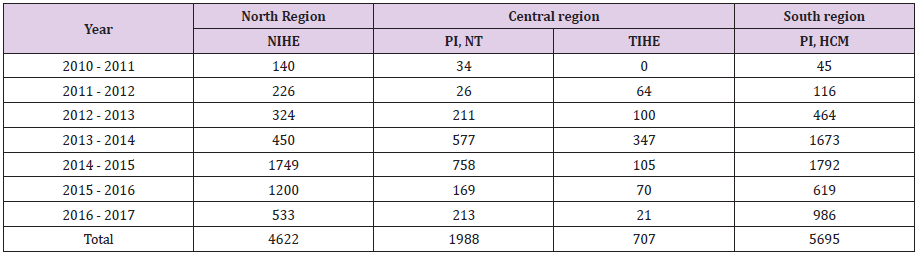
To facilitate biosafety training, four national preventive medicine institutes were authorized to provide standardized biosafety training. These are the National Institute of Hygiene and Epidemiology (NIHE), Pasture Institute Ho Chi Minh City (PIHCM), Nha Trang Pasteur Institute (NTPI), and Tay Nguyen Institute of Hygiene and Epidemiology (TIHE). A standardized 3 days (15 modules) “Laboratory biosafety” training program and training materials were developed (S File 1). Training of trainer workshop was organized to develop a cadre of biosafety trainers. The training sessions are organized at the selected institutes every month and include both theory and practical sessions. From 2010 to 2017, a total of 13,112 laboratory staff was trained. This includes 4622 staff from the north region, 2695 staff from the central region and 5695 laboratory staff from the south region (Table 3). A generic biosafety manual “Program of biosafety in the laboratory” was developed and circulated to laboratory staff as a part of the training program in the south region.
Table 3: Number of laboratory staff trained on “Laboratory Safety” from 2010 to 2017 by four authorized institutes
NIHE; National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, PI, NT:Pasture Institute, Nha Trang, TIHE; Tay Nguyen Institute of Hygiene and Epidemiology, PI, HCM: Pasture Institute, Ho, Chi Minh City
Biosafety Certification Program
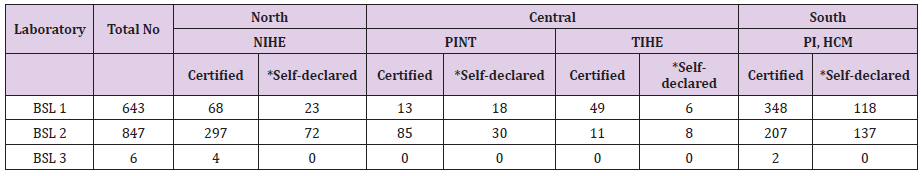
To ensure compliance to circular 29/2012/TT-BYT a laboratory biosafety certification program was established. The accreditation system was introduced in 2012 and a checklist for the assessment of BSL2 and BSL3 were developed. Since inception in 2012, 70.01% (460/657) of BSL1 and BSL2 and 100% (1/1) BSL3 in the northern region was certified (Table 4). In the South region, 64.13% (800/1263) of the BSL1 and BSL2 and 100% (2/2) BSL3 laboratories were certified. In the central region, 44% (220/406) of the laboratories were certified. In 2016, through decree 103/2016, a self-declaration of biosafety compliance has been implemented and laboratories have been encouraged to do self-declare their compliance with the biosafety standards.
Table 4: Number of biosafety certified laboratories in Vietnam.
*; Since 2016 laboratories are allowed to self-declare the biosafety compliance. NIHE; National Institute of Hygiene and Epidemiology), PINT; Pasture Institute, Nha Trang), TIHE; Tay Nguyen Institute of Hygiene and Epidemiology, PI HCM; Pasture Institute, Ho Chi Minh City
Preparedness for Outbreaks and Epidemics
To address the threat of potential emerging infectious diseases, e.g. (SARS) in 2003, human infection with influenza A (H5N1) in 2004, (MERS-CoV) and influenza A (H7N9) in 2015, Ebola in 2015, and Zika virus in 2016, Government of Vietnam established task forces to address the challenge. Experts from different ministries and departments, national hospitals/institutes (e.g. NIHE in Hanoi, Pasture Institute, Ho Chi Minh City, Hospital for Tropical Disease HCM, and Preventive Medicine Department) are included in the task force. The task force implemented disease-specific emergency preparedness program including
a. Establish “High Threat Response team” for the epidemics,
b. Issue regulation/ guidelines to address the epidemic,
c. Organize training for first-line health care workers
(nurses, doctors), and
d. Mobilize essential resources (human resources,
equipment, consumables, others).
The key to success was
e. Early recognition of the eminent or potential epidemics
threats,
f. Develop task force/working group for specific situation,
g. Developing necessary SOPs or guideline,
h. Identifying key institutes to handle the patients,
i. Rollout training programs for first responders (health
care workers, nurses and doctors), and
j. Improve diagnostic capacity [9].
Global Health Security (GHS) Preparedness
In May 2015, through Decision No: 1424/QD-BYT dated 02/05/2015 an emergency operations center (EOC) comprising of MoH departments, regional public health institutes were established. To conduct GHS drill focused on two regional public health institutes (NIHE and PI-HCMC) and EOC operation. Laboratory assessments and staff members from these two laboratories were trained on molecular diagnosis of influenza A (H7N9), enterovirus 71 (EV71), and MARS CoVI and other respiratory pathogens. In addition, mapping of the national laboratory system and strengthening the sample shipment network, testing, reporting, and referral was done. Two 3-day laboratory drill was conducted at NIHE and PI, HCM and a two-day emergency operation drill was conducted in September 2013. In the laboratory drill, panels of the pathogen (spiked samples prepared by USCDC and OUCRU) were delivered to selected laboratories and the laboratories identified the pathogens and reported to EOC. Based on the laboratory report, EOC took necessary action including resource allocation to address the epidemics, communication with international agencies and media [8]. The exercise focused on 1) effectiveness of emergency operations center (EOC) at the GDPM ; 2) improving the nationwide laboratory networking system and enhancing diagnostic capability for several priority pathogens (i.e., H5N1, MARS CoVI); and 3) creating an emergency response information systems platform.
Key Challenges
Despite relentless effort by MoH, and DoH, ensuring biosafety and biosecurity in clinical and research laboratories in Vietnam remain a challenge. Lack of awareness and compliance with the guidelines, resource limitation, limited technical support, monitoring and supervision are some of the key challenges. Developing legislation is a time-consuming process, as building consensus among experts from different fields (laboratory, food and agriculture, livestock department) need extensive discussion and justification. Lack of sufficient number of biosafety trainers to train a large number of laboratory professionals throughout the county is a challenge. Implementation of the training program was also challenging. Most laboratories could not spare staff for a 3-day long training program. Besides this, many laboratories could not afford the cost (approximately 100 US$/person) of the training. The biosafety certification program suffered from a lack of sufficient number of auditors. As a result, the DoH to implement self-assessment and reporting of the biosafety compliance.
Conclusion
Substantial progress has been made in the last seven years in implementing biosafety and biosecurity in clinical and research laboratories in Vietnam. Necessary standards, guidelines, checklists for implementing biosafety and biosecurity in laboratories have been developed. Training materials and a cadre of biosafety trainers have been developed. A joint effort by all partied including MoH, DoH, preventive medicine, and clinical laboratory management, international donors and allocation of funds would be essential for the implementation of biosafety and biosecurity standards in Vietnam.
Acknowledgement
We sincerely thank Van Ha from General Department of Preventive Medicine for the review of the manuscript. We thank Oxford University Clinical Research Unit for funding the project.
References
- Trevan T (2015) Biological research: Rethink biosafety. Nature 27(7577): 155-158.
- Sture J, Whitby S, Perkins D (2013) Biosafety, biosecurity and internationally mandated regulatory regimes: compliance mechanisms for education and global health security. Medicine, conflict, and survival 29(4): 289-321.
- International Health Regulations (2016) Organization WH. Joint external evaluation tool: International Health Regulations.
- Bakanidze L, Imnadze P, Perkins D (2010) Biosafety and biosecurity as essential pillars of international health security and cross-cutting elements of biological nonproliferation. BMC public health 10(Suppl 1): S12.
- Jennifer Gaudioso TZ (2007) Survey of Bioscience Research Practices in Asia: Implications for Biosafety and Biosecurity. Appl Biosafety 12(4): 260-267.
- Callaway E (2012) Biosafety concerns for labs in the developing world. Nature 485(7399): 425.
- Tran PD, Vu LN, Nguyen HT, Phan LT, Lowe W, et al. (2014) Strengthening global health security capacity--Vietnam demonstration project, 2013. MMWR Morbidity and mortality weekly report 63(4): 77-80.
- Wertheim HF, Puthavathana P, Nghiem NM, van Doorn HR, Nguyen TV, et al. (2010) Laboratory capacity building in Asia for infectious disease research: Experiences from the South East Asia Infectious Disease Clinical Research Network (SEAICRN). PLoS Medicine 7(4): e1000231.

 Review Article
Review Article