To the Editor
Deep vein thrombosis (DVT) is a frequent finding in patients
presenting with hip fractures [1]. Current recommendation on DVT
management with unfractionated heparin (UNH) might be difficult
and prone to complications in some circumstances. International
guidelines enable the use of fondaparinux in DVT management [2].
Fondaparinux displays some advantages in DVT management: a
single subcutaneous injection, no need for laboratory monitoring, a
reduced incidence of intolerance reactions, and no risk for Heparin-
Induced Thrombocytopenia (HIT) [2-4]. This retrospective
case series aimed to gather information about its preoperative
use in our clinic. We assessed the efficacy of the treatment
with fondaparinux, and secondary we recorded the need for
perioperative transfusion and other postoperative complications.
Data obtained from 138 records of the patients treated with 7.5
mg (5 mg in patients under 50 kg) fondaparinux (Arixtra®, Glaxo
Wellcome Production, Notre Dame de Bonneville, France), for
DVT, entered the database. The patients with DVT have had their
diagnosis established by Doppler ultrasound, and the same test
was repeated after five days of treatment, in each patient. We
registered demographic data, co-morbidities, pretreatment with
low molecular weight heparins (LMWH), dose and duration of
treatment with fondaparinux, time to surgery after cessation of
fondaparinux, type of anesthesia, the in-hospital mortality, and the
hemorrhagic events. The amount of blood transfusion (number of
units transfused) until the fourth postoperative day was recorded.
The transfusion criteria were hemoglobin < 7g/l or hemodynamic
instability in circumstances of blood loss. In patients with
coronary heart disease, the targeted hemoglobin level was 10g/l.
In patients with persistent DVT after fondaparinux administration,
the subsequent treatment applied was recorded as well as the
complications if these occurred.
Data were expressed as mean, standard deviation (SD), median
(range), numbers and percentiles. Out of 138 patients’ files, four
were dropped out because of lacking information, and a remaining
134 of medical records entered the study analysis. Patients’ age was
between 24 and 94 years with a median of 75 years, 53 (39.5%) were
male and 81 (60.5%) female. The demographic data and risk factors
for DVT are presented in Table 1. One hundred and eight patients
(80.5%) with DVT who received fondaparinux have been gone
further with surgery, and 98 patients received spinal anesthesia
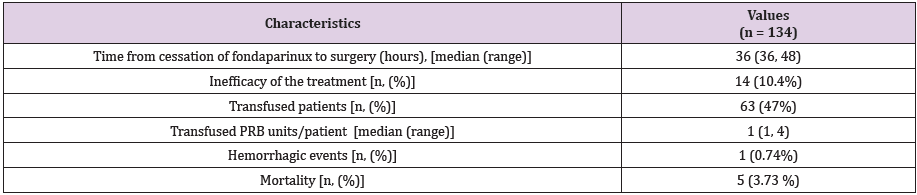
and 10 general anesthesia. We did not record any evidence of
heparin-induced thrombocytopenia, major bleeding, or spinal/
epidural hematoma. In Table 2 we registered data regarding the
treatment with fondaparinux and its complications. Fondaparinux
was efficient in 89.6% of cases, to treat venous thrombosis.
There is a lack of literature regarding the preoperative use of 7.5
mg fondaparinux for DVT, even if there are confirmations on its
efficiency and safety, in various situations [5,6]. Our study showed
efficacy and safety with the preoperative use of fondaparinux, even
if regional anesthesia was used. The above data have been collected
in only one single medical center and there is a need for further
studies to validate our findings.
Table 1: Demographic data and risk factors in studied patients (n=134).
SD - standard deviation; DVT - deep vein thrombosis; n - number of patients.
Table 2: The fondaparinux treatment characteristics and complications.
PRB- packed red blood cells, SD - standard deviation, n - number of patients.
References
- Bengoa F, Vicencio G, Schweitzer D, Lira MJ, Zamora T (2018) High prevalence of deep vein thrombosis in eldery hip fracture patients with delayed hospital admission. Eur J Trauma Surg.
- Kearon C, Kahn SR, Agnelli G, Goldhaber S (2008) American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. In Kearon C, Kahn SR, Agnelli G, Goldhaber S (Eds.), (8th)., Chest 133(Suppl 6): 454S-545S.
- Garwood CL, Gortney JS, Corbett TL (2011) Is there a role for fondaparinux in perioperative bridging? Am J Health Syst Pharm. 68(1): 36-42.
- Büller HR, Davidson BL, Decousus H (2004) Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: a randomized trial. Ann Intern Med 140(11): 867-873.
- Nagler M, Haslauer M, Wuillemin WA (2012) Fondaparinux - data on efficacy and safety in special situations. Thromb Res 129(4): 407-417.
- Schindewolf M, Scheuermann J, Kroll H (2012) Application, tolerance, and safety of fondaparinux therapy in a German hospital: a prospective single-centre experience. Thromb Res 129(1): 17-21.

 Letter to Editor
Letter to Editor