Abstract
A cross-sectional study was conducted in Honkolowabe district, East Arsi zone, from November, 2017 to March, 2018 with aim of determining the prevalence of ovine lungworm infection, to assess associated risk factors and identification of species of lungworm which circulate in study area by using coproscopic examination. In this study faecal samples were randomly collected from 384 sheep from different PAs. Age groups, sexes, body conditions and respiratory signs were used as risk factors. Then, (L1) were extracted by Modified Baerman technique and examined under low magnification power of Compound Microscope.The finding indicate that 259 (67.4%) were infected with different species of lungworm; namely, Dictyocaulusfilaria (40.1%), Protostrongylusrufescens (15.9%), mixed infection (6.8%) and Muellerius capillaries (4.7%). There were statistically significant difference (p<0.05) in the prevalence of lungworm with regard to body conditions(poor 76.5%, medium 68.4%, good 55.1%) and manifestation of respiratory signs (83%, shown respiratory sign and 51% didn’t show). 69.8% females and 64.8% male; 70.3% young and 66% adult were infected with one or more species of parasite. In conclusion, this study revealed that lungworm is one of the major respiratory parasites that affect the health and productivity of sheep in Honkolowabe; that needs attention for its control and prevention to reduce the currently reported high prevalence.
Keywords: Honkolowabe; Lung Worm; Prevalence; Risk Factors; Sheep
Introduction
Ethiopia is known by having the highest number of livestock
population. According to the recent estimate of livestock population
of the country, Ethiopia is a home for about 59.5 million cattle, 30.7
million sheep, 30.2 million goats, 12.22 million equines and 56.5
million poultry CSA [1]. About 99.8% of the sheep and nearly all
goat population of the country are local breeds. Of the total sheep
population, 75% are raised in highlands with altitudes above
1,500 meter above sea level CSA [2]. Sheep and goats are the most
numerous of man’s domestic livestock. In Ethiopia, sheep are the
dominant livestock providing up to 63% of cash income and 23% of
food substance value obtained from livestock production Bogale, et
al.[3,4] It is estimated that 1, 078, 000 sheep and 1, 128, 000 goats
are used in Ethiopia for domestic consumption annually Alemu,
et al. [5]. However, the productivity is much less when compared
with the population size of small ruminant in Ethiopia Fentahun,
et al. [6] and the economic benefits to the farmers remain marginal
due to prevailing disease, poor nutrition, poor animal production
systems and general lack of veterinary care Sissay et al. [7].
Helminth parasites are among the causes of substantial
productivity losses in ovine production of the country by causing
respiratory diseases resulting in great economic concern in the
highlands of Ethiopia where sheep are important livestock units
Basaznew, et al. [8] Sheep Lungworm disease are one of the major
respiratory disease that widely distributed throughout the world
but are particularly common in countries with temperate climate
and in the high land of tropical and sub- tropical countries of
the world providing nearly perfect condition for their survival
and development.Lung worm infection is also called Verminous
Bronchitis or Verminous Pneumonia which caused by the three
economically important species of lungworm of sheep and
goat namely; Dictyocaulusfiaria, Protostrongylusrufescens and
Muellerius capillaries Abebe, et al. [9]. These nematode parasites belong to two super family, Trichostrongyloidea (D. filaria) and
Metastrongyloidea (P. rufescens and M. capillaries). Its pathogenic
effect depends on their location within the respiratory tract, the
number of infective larvae ingested and the immune system of the
animals. Protostrongylidae species occur in the alveoli, bronchioles
and parenchyma of the lungs of various species of mammals Umur,
et al. [10].
Dictyacaulusfilariahas a direct life cycle whereas M. capillaries and P. rufescens have indirect life cycles. Dictyacaulus filarial infection is acquired by ingestion of infective larvae with herbages but M. capillarysand P. rufescens are transmitted when intermediate hosts are accidentally ingested by grazing animals. Dictyacaulusfilariais the most important lungworm of sheep and goats and commonly associated with a sign of coughing and unthriftiness, which usually affects lambs and kids. Muellerius capillaries and P. rufescens are more common but less pathogenic when compared to D. filarial Radostits, et al. [11]. The clinical sign of infected animals can be less obvious than signs of other livestock diseases Tamire, et al. [12]. However, in naturally affected animals clinical signs like reduced growth, loss of appetite, increase respiratory rate and coughing are usually observable Shite et al. Prevention and control of these parasites is critical to enhance the economic benefit from these species of livestock Alemu, et al. [13]. In Ethiopia, although fragment studies have been done and reports are available, however, little information has been documented on the status of ovine lungworm infection. Moreover, no information has been documented on the status and associated risk factors of the disease in ovine in Honkolowabe district.
Therefore, this study was aimed to;
1. Determine the prevalence of lungworm infection in sheep
population in selected areas of honkolowabe district
2. To identify risk factors and quantify their degree of
association with the disease
Materials and Study Methodology
Study Area
The study was conducted from November 2017 to April 2018 in 5 selected peasant association(PA), Bekojimirx, Changicha, Siltana 01, Machitulaman and Tajiwalkite of HonkoloWabe district, East Arsi zone, Ethiopia. It lies East of Wabe River, which separate East Arsi zone from Bale zone and it is located, 269 km East of Addis Ababa. The area receives an annual range of rain fall from 1200- 2150mm and has a biomodal rainfall occurring from March to April (a short rainy season) and from July to October (long rainy season). Topographically, it is situated at altitude of 2200 - 3850 m above sea level with the mean annual minimum and maximum temperature 18 °C and 20%, respectively. This area is characterized by mixed farming system, which is engaged in agricultural and livestock production HWWADO [14]. Honkolowabe district has 9 peasant Associations and 2 town kebeles and of these the study was done in randomly selected 5 representative peasant Associations.
Study Population
The estimated animal population in the area was about 87216 cattle, 17994 sheep, 9094 goats, 9360 horses, 9520 donkey and 39250 chickens HWWFLO [15]. The study animals, sheep, in selected peasant association were maintained under extensive management system in small household flocks of mixed age groups and sexes with no supplementary feed. All animal in selected peasant association were indigenous (local breeds). Sheep with age group of young and adult from both sexes, with different body condition, with clinical respiratory signs and those that appeared apparently healthy was included in the study. The age of every sampled sheep was categorized in to young and adult using dentition and information from the owners. Age was determined using dental eruption formula into two age categories (Greater than 2 years and less than two years) Steel [16] and by asking the owner’s. The body conditions score of study animals was divided into good, medium and poor based up on their criteria of body score Abebe [9].
Study Design, Sampling Strategy and Sample Size Determination
A cross sectional study design was selected to determine the prevalence of lung worm in the study area, the predominant species of lungworm and to evaluate the effect of major host related risk factors on the occurrence of the parasites in 5 peasant Associations of Honkolowabe district. The samples were collected by using simple random sampling method to select the study animals in the area. Since the prevalence of ovine lung worm in HonkoloWabe district has not been reported, 50% expected prevalence rate, 95% confidence interval and 5% desired absolute precision was used Thrustfield [17]. Therefore, the total sample size required was 384 based on the given formula below. Where, n = required sample size

Pexp = expected prevalence
d = desired absolute precision
Sample Collection and Laboratory Analysis
Faecal samples were collected directly from the rectum of selected animals in universal bottle. While collecting faecal sample; sex, age, body condition and respiratory signs are properly recorded on the prepared format. Specimens were transported to Asella regional veterinary laboratory in universal bottles under airtight conditions within 4 h. In the laboratory, faecal sample examination for the presence of L1 larvae was conducted using modified Baermann technique. Briefly 5gm to 10gm of fecal material was wrapped in double layered gauze and suspended in conical flask containing warm water (approximately 40 °C) using a clip wire. The whole apparatus was left for 24 h overnight. So that the larvae left the faeces and migrated through the gauze and settled at the bottom of the glass Urquhart, et al. [18] Then after, the wrapped feaces were removed and the supernatant was discarded from the conical flask gently. After that, a small amount (3-5 ml) of sediment was transferred to a watching glass using Pasteur pipette for examination of L1 under low power of the microscope. A drop of 1% iodine solution was added to the positive sample to immobilize the larvae for the identification of species Urquhart, et al. [18] All larvae were indentified morphologically as described by previous workers Hansen, et al. [19]. Then, the result was recorded on the prepared format.
Data Management and Analysis
The raw data was entered and managed using Microsoft Excel worksheet and summarized with descriptive statistics. Statistical Package for Social Science (SPSS) software version 20 was used to determine the prevalence of the disease and the association between risk factors and the disease. The association between prevalence and risk factor was assessed by using Pearson’s Chi- Square. A statically significant association between variables was considered to exist if the computed P-value is less than 0.05.
Results
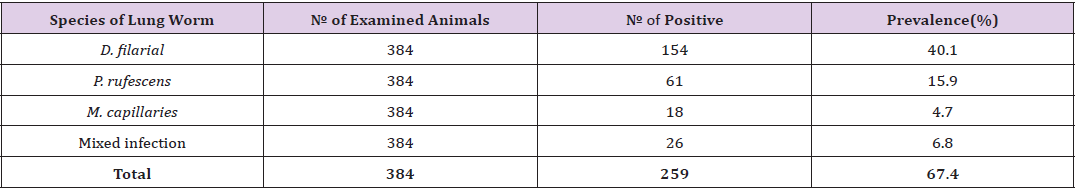
The overall prevalence of ovine lung worm in the study area was 67.4%. The result revealed that of total 384 fecal samples examined 259(67.4%) were found positive for lung worms with different species of lungworm. Out of these 40.1%, 15.9%, 4.7% and 6.8%, was due to D.filaria, P.rufescens, M.capillaries, and mixed infection, respectively. Thus, D. filarial was the most dominant species followed by P.rufescens. M. capillaries were the least, and certain animals were infected by mixed infection (Table 1). In this study, variables such as PAs, sex, age, respiratory signs and body condition were considered to be risk factors for the occurrence of lungworm in sheep. The findings on these factors and association between the prevalence of the disease and the risk factors were as presented here below.
Prevalence of Lungworm Infection According to Peasant Association
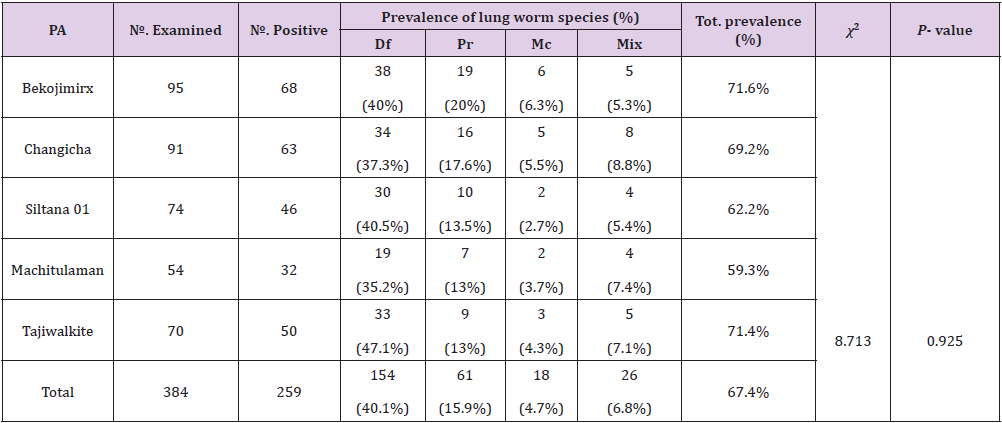
The prevalence of lungworm in selected PA showed that 71.6%, 69.2%, 62.2%, 59.3% and 71.4% in Bekojimirx, Changicha, Siltana 01, Machitulaman and Tajiwalkite respectively. The highest prevalence was observed in Bekojimirx (71.6%) and the lowest prevalence was observed in Machitulaman (59.3 %). There was no significant difference (P>0.05) in the prevalence of lung worms among the kebeles (Table2).
Table 2: Prevalence of ovine lung worm in the selected study area.
Note: PA =Peasant association, Mix= Mixed infection, Df= Dictocaulus filarial, Pr= Protostronglus rufescens, Mc= Mullerius capillaries, P-value= Pearson’s value, Tot.= Total
Prevalence of Lungworm Infection According to Age of Study Animals
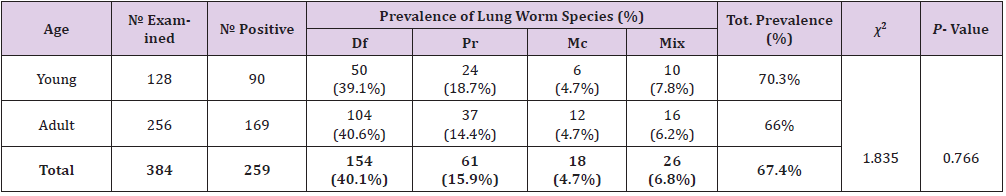
The prevalence of lungworm infection according to age of study animals was higher in young sheep (70.3%) than in adult sheep (66%), however, the difference was not statistically significant (p>0.05) (Table 3).
Table 3: Prevalence of Lungworm Infection According to Age of Study Animals.
Note: Mix= Mixed infection, Df= Dictocaulus filarial, Pr= Protostronglus rufescens, Mc= mullerius capillaries, P-value= Pearson’s value, Tot.= Total, χ2 = chi-square
Prevalence of Lungworm Infection According to Sex of Study Animals
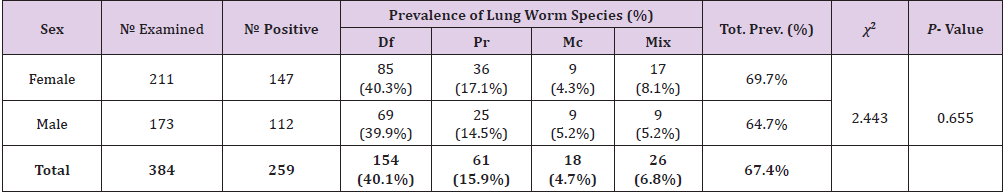
The prevalence of lung worm in both sex’s shows that from 173 males examined 112 (64.7%) and from 211 females 147 (69.7%) were positive for lung worm. Although the prevalence of lung worm was relatively higher in females than male individuals, no statistically significant difference was observed among them (P>0.05) (Table 4).
Table 4: Prevalence of Lungworm Infection According to Sex of Study Animals.
Note: Mix= Mixed infection, Df= Dictocaulus filarial, Pr= Protostronglus rufescens, Mc= mullerius capillaries, P-value= Pearson’s value, Tot.= Total, χ2 = chi-square
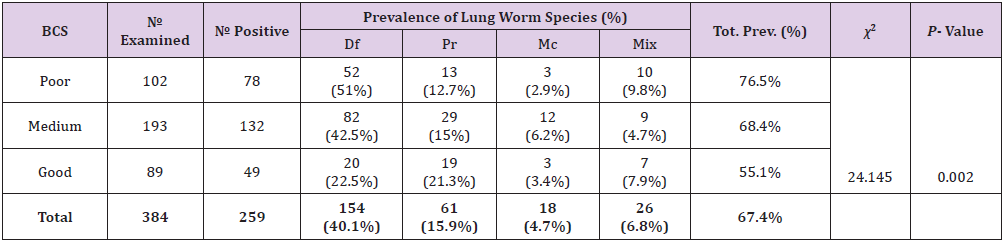
Prevalence of Lungworm Infection According to Body Condition of Study Animals
Prevalence of lungworm infection according to body condition of study animals was 76.5%, 68.4%, and 55.1% in poor, medium, and good, respectively. In this study, animals having poor body condition were found to have higher prevalence to ovine lungworm infection than moderate and good body conditions and the difference among the different body condition categories was statistically significant (p<0.05)(Table 5).
Table 5: Prevalence of lungworm infection according to body condition of study animals.
Note: BCS =Body Conditions, Mix= Mixed infection, Df= Dictocaulus filarial, Pr= Protostronglus rufescens, Mc= mullerius capillaries, P-value= Pearson’s value, Tot.= Total χ2= chi-square
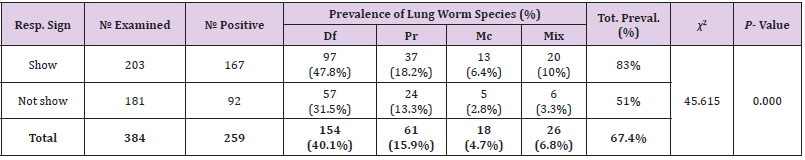
Prevalence of Lungworm Infection According to Manifestation of Respiratory Signs
Prevalence of lungworm infection in study animals according to manifestation of respiratory signs (coghing and nasal discharge) was 82.3% and 51% in apparently healthy sheep, respectively. The difference between the prevalence of lungworms in animals showing respiratory signs and in animals which were apparently healthy was statistically highly significant (p<0.05) (Table 6).
Table 6: Prevalence of lungworm infection according to manifestation of respiratory signs of study animals.
Note: Resp.Sign =Rspiratory sign Mix= Mixed infection, Df= Dictocaulus filarial Pr= Protostronglus rufescens, Mc= mullerius capillaries, P-value= Pearson’s,value, Tot.= Total χ2=chi-square, Preval.=prevalence
Discussion
The present study result indicated that lungworm infection
was one of the most common respiratory diseases of sheep with an
overall prevalence of 67.4%. This result is almost in agreement with
research findings that were conducted in Munesa district, 66.67%,
by Abdella, et al. [20], Debra Tabor Awraja, 70.7%, by Yohannes
[21], Asella, 59.9%, by Hussein, et al. [22], Bassona Worena district,
62.67%, by Zelalem [23], Debra Birhan, 64.7% by Brook, et al.
[24] However, the current finding was relatively lower than the
prevalence reported by Jovanovic, et al. [25] in highland of Shoa
province 83 to 99.5%, Eyob, et al. [26] in Asella province, 72.44%,
Netsanet [27] in DebreBirhan, 73.75% and Sefinew [28] in six
district of Wollo, 71.3%. The current finding was higher than study
conducted by Ibrahim, et al. [29] in Mekeletown, 13.4%, Mekonnen,
et al. [30] at Gonder, 32.7%, Regassa, et al. [31], in Dessie and
Kombolcha districts, Northern Ethiopia, 40.4%. The variation in the
overall prevalence rate in those areas might be due to differences in
nutritional status, level of immunity, management practice of the
animal, rain fall, humidity and temperature differences and season
of examination which may favor / disfavor the survival of parasite
larvae on their respective study area Kebede, et al. [32]
In current study, the prevalence of different species of lungworm
was 40.1%, 15.9%, 4.7% and 6.8% due to D. filaria, P. rufescens,
M. capillaries, and mixed infection with two or three species of
lungworm, respectively. It was observed that D. filarial was the most
predominant species in the area followed by P. rufescens, whereas
M. capillaries were the least prevalent. This finding is supported by
Mihreteab, et al. [20,22,33] who reported D. filarial to be the most
prevalent in their study. In contrast to these findings, Sisay [34] in
Bahir Dar and Basaznew, et al. [8], in Dessiezuria district reported
that M. capillaries were the most prevalent in their studies. The
possible reason for the predominance of D. flaria in the study
area might be attributed to the difference in the life cycles of the
parasites. Thus, D. flaria has a direct life cycle and requires shorter
time to develop to an infective stage. After ingestion, the larvae of
these parasites can be shed with feces within 5 weeks Soulsby [35].
Unlike D. flaria the transmission of P.rufescens and M. capillaries
needs an intermediate snail for completing its life cycle.
In addition to this the low prevalence of both M. capillaries and
P.rufescensin the study area might be attributed to the fact that the
study was done in dry season which does not favor the development
of the snail inter mediate hosts Kahn [36]. Mixed infection was also
observed in the current study as in many previous studies Poulos
[20,22,37]. On the attempt to assess the influence of the study area
on lung worm infection of sheep, Insignificant effect (P> 0.05) was
observed on the prevalence of lung worm infection. The highest
prevalence (71.6%) was observed in Bekojimirx followed by
Tajiwalkite (71.4%) and the lowest in Machitulaman (59.3%). The
reason might be associated with the time of sampling as Samples
were not collected at the same month from the study area. This
insignificant difference agrees with the report of Desta, et al. [38],
in Ambo Wondwossen, 1992, in and around Asella. In contrary to
the present study, a significant difference has been reported by
Alemu, et al. [5,22]
These differences between researchers might be associated
with variation in sample size, duration of study time, difference
in agro-ecology and season of study period. Highest prevalence in
Bekojimirxmight be due to it has relatively low temperature, higher
moisture and humidity than other ecologies which is favorable
for survival and development of larvae and intermediate hosts
Radostits, et al. [39] An attempt was made to know the influence
of age on the prevalence of lungworm infection and there is no
statistically significant difference (P>0.05) in the infestation rate
of lungworm. However, a high infection prevalence of lungworm
was recorded in younger (70.3%) than an adult (66%) which is in
agreement with the observations of Eyob, et al. [26,33]. This might
be associated with the apparent inability of the host to develop
acquired immunity. Relatively, adult animals have more frequent exposure to lungworms than young animals; so that more likely to
develop acquired resistance to these specific parasites or recovered
animals have better immunity against re-infection Soulsby [35].
In this study, attempts were made to know if there is variation in lung worm infection between different sex groups. The prevalence was higher in female (69.7%) than male (64.7%), but the difference was statistically insignificant. This agrees with research reported by Addis, et al. [22,26,40], but disagree with report of Alemu, et al.[13,33]. These differences between researchers might be either due to improper distribution of sample selection between e sampled or most of the sampled females are not in preparturient period during the study time that make both sexes equally susceptible to disease. In current finding prevalence was higher in female; this might due to certain sampled animal were lactating which suppress immunity of the animal Urquhart, et al. [18] Higher infection prevalence was recorded in sheep showing clinical respiratory sithe two sexes that makes prevalence higher in female Addis, et al. [40] where almost all female sheep wergns (83% than those apparently healthy (51%). Hence, the variation was statistically significant (P<0.05). The result coincides with the observation of Paulos [22,26,37].
Even though apparently healthy sheep show low infection compare to those showing clinical respiratory signs groups, about 51% of them were infected with lungworm. The reason might be due to the parasites were in pre-patent stage or due to small adult worm burden in sheep which couldn’t produce eggs and hence larvae; or as a result of immunity developed due to exposure to a few lungworms which is not associated with clinical sign but animal shed larvae Soulsby [35]. About 17.7% of those animals manifesting respiratory signs were appeared negative on examination; this might be, due to bacterial or viral diseases that may causes occurrence of respiratory signs Gelagay, et al. [41] With regard to body condition, the prevalence of lungworm infection was higher (76.5%) in animals of poor body condition than those of medium (68.4%) and good (55.1%). The difference among body condition was statistically significant (p<0.05). This finding agrees with study reported by Muhammed, et al. [23,42] that reported high prevalence in animals with poor body conditions. This might be associated with the nutritional management of animals. The possible explanation for this observation could be due to immunesuppression in sheep with poor body conditions, concurrent infection by other parasites including gasstrointestinal helminthes and malnutrition which leads to lack of resistance to infection and contribute for increased prevalence. Evidently, the infection with a parasite by itself might results in progressive emaciation of the animals Radostits, et al. [31,43, 44]
Conclusion and Recommendations
Lung worms are one of the most common causes of respiratory problem in Ovine. The result of present study indicated that prevalence of lung worm was high in Honkolowabe district, East Arsi zone. The Coproscopical examination indicated that 67.4% of the examined sheep were infected with different species of lungworms. Sheep with poor body condition and those with clinical respiratory signs were highly significantly infected with either of the lungworm species than their counterparts. Out of the recovered lungworms (D. filarial, P.rufescens, M. capillaries and mixed), D. filarial was found to be the highly prevalent lungworm of ovine’s in the study area. The high prevalence of lungworm infection in the study area revealed the need much attention in the control and prevention of these parasites.
In view of the above facts, the following recommendations are forwarded;
a. Regular deworming with effective antihelmintic should
be routinely practiced in the area
b. Supplementation of additional feed to make sheep well
nourished and good body condition.
c. Emphasis should be given to the control and prevention in
order to reduce the prevalence from the current high finding
d. Further research should be carried out in this study area
since the prevalence of the diseases was most significant.
e. Veterinary service provider in the area should have the
facility to diagnose and treat ovine lung worm infected animals
to mitigate production losses.
References
- (2017) CSA Agricultural sample survey 2016/2017 (2009 EC).Volume III. Report on livestock and livestock characteristics. Statistical Bulletin 585, Addis Ababa, Ethiopia 9-20.
- (2013) Central Statistical Agency (CSA): Report on Livestock and livestock characteristics (Private peasant holdings). Statistical Bulletin. Addis Ababa 2: 570.
- Bogale B, Ebre A, Melaku A (2012) Ovine Lungworm Infection: Prevalence, Species Composition and Associated Risk Factors in DessieZuria District, Northeastern Ethiopia. African Journal of Basic and Applied Sciences 4: 73-76.
- Ibrahim N, Godefa Y (2012) Prevalence of Ovine Lung Worm Infection InMekelle Town, North Ethiopia. The Internet Journal of Veterinary Medicine 9 (1): 1-7.
- Alemu, Y, Merkel RC (2008) Sheep and Goat Production Hand Book for Ethiopia, Ethiopian Sheep might and Goat Productivity Improvement Program (ESGPIP). p. 2-6.
- Fentahun T, Seifu Y, Chanie M, Moges N (2012) Prevalence of Lungworm Infection inSmall Ruminants in and Around Jimma Town, Southwest Ethiopia. Global Veterinaria 9(5): 580-585.
- Sissay M, Uggla A, Waller PJ (2007) Epidemiology and seasonal dynamics of gastrointestinal nematode infections of sheep in a semi-arid region of eastern Ethiopia. Journal of Veterinary Parasitology 143: 311-321.
- Basaznew B, Ayalew A, Achenef M (2012) Ovine Lungworm Infection: Prevalence, Species Composition and Associated Risk Factors in DessieZuria District, Northeastern Ethiopia. Afr J Bas and Appl Scie 4(3): 73-76.
- Abebe R, Melesse M, Mekuria S (2016) Lungworm Infection in Small Ruminants in and Around WolaitaSoddo Town, Southern Ethiopia. Journal of Veterinary Science & Technology 7: 302.
- Umur S, Karol E, Guclu F, Tinar R (2006) Nematodes. InHelmintology. pp. 214-449.
- Radostits O, Gay C, Hinchcliff K, Constable P (2006) Veterinary Medicine. A Text book of the disease of cattle, horses, sheep, pigs and goats (10th), Bailler Tindal, London, USA. pp. 1568-1569.
- Tamire K, Mohamed A (2013) Prevalence of Ovine Lung Worms- Around Bahir Dar, East Africa, Ethiopia. Acta ParasitologicaGlobalis 4(3): 71-79.
- Alemu S, Gelay E, Ayele G, Zeleke A (2006) Study on small ruminant lungworm in North East Ethiopian. Egyptian J Vet Parasitol 14(2): 330-335.
- (2015) HonkoloWabeWoreda Agricultural Development Office (HWWADO).
- (2016) Honkolowabeworeda fish and livestock office (HWWFLO).
- Steel M (1996) Goats: The tropical agriculturalist. London and Basing Stoke Macmillan Education Ltd. ACCT. 79-83. Abebe G (2007) Body condition score of sheep and goats, Ethiopian sheep and goats production improvement program (ESGPIP) 3.
- Thrusfield M (2005) Veterinary epidemiology, (2nd), UK: Blackwell Science. pp. 178- 187.
- Urquhart HM, Armour J, Duncan J, Dunn AM, Jennings FW, et al. (1996) In Veterinary parasitology. (2nd), London: Black well science Ltd. p. 33-60.
- Hansen J, Perry B (1994) The epidemiology, Diagnosis and control of Helminthes parasites of ruminants, ILRAD, Kenya. p. 29-31.
- Abdella S, Abdela A, Abduselam A, Daniel B, Fikre N, et al. (2016) Prevalence Of Ovine Lungworms In Munesa District, East Arsi, Ethiopia 8(7).
- Yohannes G (1989) Epidemiological study and Antihelmentic trail of Ovine Dictyocaulasisin Debretabor Awraja, DVM thesis, Faculty of Veterinary Medicine, Addis Ababa University Debrezeit 81.
- HussenY, Jabir A, Seifudin K, Mukarim A (2017) Study on Prevalence of Ovine Lungworm in Goba District, Bale Zone, South East Ethiopia. International Journal of Research Studies in Biosciences (IJRSB) 5(7): 37-47.
- Zelalem Y (2011) Lung worm, infection in ovine prevalence and associated risk factors in Basona and Worran district, BSC Thesis, FVM, University of Gondar, Gondar, Ethiopia.
- Brook L, Fesseha G, Shibru T (1986) The seasonal occurrence of Dictyocaulusfilaria (Rudophi.1800) in four selected sites of Ethiopia 9: 25-38.
- Jovanovic M (1962) Observation of D. filaria infection in and around Shoa, a report to the ministry of Agriculture, Addis Ababa, Ethiopia. p. 12.
- Eyob E, Matios L (2013) The prevalence and risk factors associated with Ovine lungworm infestation in the Asella province, Central Ethiopia. J Parasitol Vector Bio 5: 118.
- Netsanet B (1992) Study on prevalence and control of lungworm in local Ethiopian highland sheep in and around Deberebirhan. DVM Thesis, FVM, AAU, Debrezeit. of Internal Parasites in Sheep and Goats. Technical Bulletin No.3.Oregon State University. 9055: 1-4.
- Sefinew A (1999) Survey of small ruminant lungworm in six district of Wollo. DVM Thesis, FVM, AAU, Debrezeit.
- Ibrahim N, Degefa Y (2012) Prevalence of Ovine Lung Worm Infection in Mekelle Town, North Ethiopia. Int J Vet Med 9: 1-15.
- Mekonnen A, Abebe F, Yohannes E (2011) Study on the Prevalence of Lungworm Infection in Small Ruminants in Gondar Town, Ethiopia. Vet Res 4(3): 85-89.
- Regassa A, Toyeb M, Abebe R, Megersa B, Mekibib B, Mekuria S, et al. (2010) Lungworm infection in small ruminants:Prevalence and associated risk factors in Dassieand Kombolcha districts, northeastern Ethiopia.Vet Parasitol 169(1-2): 144-148.
- Kebede S, Menkir S, Desta M (2014) On farm and Abattoir study of Lungworm infection of small ruminants in selected areas of Dale District, Southern Ethiopia. Int J Curr Microbiol App Sci 3: 1139-1152.
- Mihreteab B, AmanA (2011) Ovine Lungworms in Tiyo District, South-East Ethiopia: Prevalence, Effect of Altitude and Major Host Related Risk Factors. Global Vet 7(3): 219-225.
- Sisay A (1996) Prelimary study on the prevalence of ovine lung worm infection in and around Bahir Dar, DVM thesis, FVM, Addis Ababa University, Debrezeit, Ethiopia.
- Soulsby EJL (1982) Helminthes, Arthropods and Protozoa of domesticated animals, (6th ), Bailliare Tindal, London 492.
- Kahn C (2005) The Merck Veterinary Manual. Ninth Edn. Merck and co., INC., white house station, USA. pp. 215-256.
- Paulos A (2000) Importance of Seasonal Dynamics of Lungworms infection of Small Ruminants in Chilalo areas, Arsi Zone. DVM Thesis, FVM, AAU, Debrezeit, Ethiopia.
- Desta B, Sisay N, Dinka A, Fufa A (2010) The prevalence of Lungworms in Naturally Infected Sheep of Ambo District, Oromia, Ethiopia. Global Veterinaria 10(1): 93-98.
- Radostits OM, Gay C, Blood DC, Hinchclift KW (2007) Veterinary Medicine: A Text Book of the Diseases of Cattle, Sheep, Goats, Pigs and Horses. (10th Edn). London. Harcourt Publishers. pp. 1541-1564.
- Addis M, Fromsa A, Ebuy Y (2011) Study on the prevalence of Lungworm Infection in SmallRuminants in Gondar Town, Ethiopia. J Anim Vet Adv 10: 1683-1687.
- Gelagay A, Laekemariam Y, Esayas G, Selam T, Kassahun A, et al. (2004) Epidemiologic and Serologic Investigation of Multifactorial Respiratory Disease of Sheep in the Central Highland of Ethiopia. Intern. J Appl Res Vet Med 2: 274-275.
- Muhammed H, Alebachew T, Ayichew T, Destaw E (2016) Study on Lungworm and Associated Risk Factors of Small Ruminants in HitosaWoreda, Ethiopia, WolaitaSodo University, School of Veterinary Medicine, Ethiopia. Global Veterinaria 17: 303-309.
- Wondwossen T (1992) Prevalence of lungworm in and around Asella, DVM thesis, Faculty of Veterinary Medicine, Addis Ababa University Debrezeit.
- Hasen A, Tekele S, Simenew K (2013) Ovine lungworm infestation rate on fecal larvae recovery basis. Acta Parasitol Glob 4: 29-33.

 Research Article
Research Article