Abstract
Natural components thought to have a particular benefit nowadays. They are playing a necessary role in health attention. Sea cucumber is theorized to be a significant source of bioactive components. Moreover, Flavonoid glycosides are a group of polyphenols with different glycoside substituent that possesses diverse pharmacological activities and can help management coronavirus. So, this study aimed to extract saponin and polysaccharides from sea cucumber, also, extract both of naringin, hesperidin from orange peel and convert hesperidin to hesperetin then chemical analysis of all extracted compounds was performed to confirm their structure and evaluate their antioxidant, antibacterial and antitumor activity. The concentrations of saponin, naringen, hesperidin, hesperetin, and ascorbic acid, which scavenged 50% of DPPH radicals, were 10.50, 0.13, 0.13, 0.66, and 0.0025 mg/ml respectively. Furthermore, Cell viability of saponin, hesperidin and hespertin showed a growth inhibitory effect, IC50 28.78, 236.40 and 73.99. The obtained data indicated that sea cucumber saponin, polysaccharides, and orange peels, may provide a promising new therapeutic approach to HEPG2 cancer cells. Also, these compounds were effective antioxidant, so they may be effective and scavenge free radicals which resulted from the disease.
Keywords: Sea cucumber; Saponin; Polysaccharide; Naringin; Hesperidin; Hesperitin
Abbreviations: FT-IR: Fourier Transforms Infrared Spectroscopy; UV: Ultraviolet Spectra; XRD: X-ray Powder Diffraction; TGA: Thermal Gravimetric Analysis; DPPH: 2, 2-Diphenyl-1-Picrylhydrazyl-Hydrate; MTT: 3-4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide.
Research Highlights
a) Saponin (holothurians) and polysaccharide have been
extracted from sea cucumber.
b) Orange peel was treated with a different solvent to extract
Naringin, Hesperidin and hespertin.
c) All of the extracted compounds were chemically analyzed
to confirm by chemical analysis and evaluated their antioxidant,
antibacterial and antitumor activity.
Introduction
Natural components thought to have a particular benefit
nowadays [1]. They are playing a necessary role in health attention
and more probable to produce a pharmacologically effective
component, that act as an ingredient in artificial drugs [2]. Marine
sea cucumber having several active components [3], distinguishing
by their nutritional value [4]. Marine invertebrates, sea cucumber
own a worthy bioactive ingredient, for instance, holothurians that
exhibited biological effectiveness and have a therapeutic effect [5].
Furthermore, it holds more than 50 forms of nutrients inclusive
amino acids, polyunsaturated fatty acids, vitamins, and trace
element and active substances such as polysaccharides, proteins
and glycosides [6]. Saponin which is a bioactive compound that
exist in large amounts in both marine sea cucumbers and sponges,
and it has a difference of biological and pharmacological effect [7],
as antitumor, anti-bacterial, anti-inflammatory and hypoglycemic
agent [5,8].
Polysaccharides have numerous activities, for example, antitumor,
immune promoting, and antioxidant, polysaccharides
are one of the remarkable ingredients of natural compounds
[9]. Citrus juice remains are at most composed of peel, juice, and
seeds. The peel composed of bioactive compounds like those
flavones [10]. They are consisting of high bioactive components
inclusive flavonoids, limonoids and glycosides [11]. Citrus peels,
seeds and fruit pulps, that account for 50% of the original whole
fruit mass, are a by-product of the juice, marmalade and canning
manufactures [12]. Both naringin and hesperidin that consider
the main citrus flavonoids which found in orange extraction and
at most in grapefruit and sour oranges [13]. These flavonoids have
been found in the serum of people after eating or drinking orange
and grapefruit [14]. Hesperidin considers a bioactive compound
in preventing numerous diseases, for example, lowering capillary
permeability, and anti- inflammatory, antibacterial and antitumor.
Hesperidin Furthermore has the ability to monitor liver cholesterol
texture by repressing the activity of 3-hydroxy-3-methylglutaryl
coenzyme A reductase [15].
Hesperidin is functionally utilized as a supplement factor in
the therapy protocols of complementary settings. Its deficiency has
been related to abnormal capillary leakiness as well as the ache
in the extremities, which cause pain, impairment and leg cramps.
Supplemental Hesperidin also helps in decreasing edema which
a result of fluid accumulation. Also, it has the ability to manage
corona virus COVID-19 [16,17]. Naringin displayed the capacity
of an antioxidant [18]. Moreover, it considers anti-inflammatory agent [19,20], anti-breast cancer agent [21], anti-allergic [22], and
hypoglycemic compounds [23]. Naringin has been considering the
anti-angiogenesis agent [24]. Also, naringin might be the active
ingredient in suppressing osteoclastogenesis and osteoclasts in
both vitro and vivo model [25]. Furthermore, naringin has the
ability to repress polymethyl methacrylate particles induced
osteolysis in vivo [25].
Materials and Methods
Saponins Extraction
Triterpenoid saponin has been extracted from marine sea cucumber, Holothuria thomasi, (Red Sea, Egypt, identified by Zoology department, Faculty of science, Tanta University), according to [27]. Dried body walls of marine invertebrates see cucumber were grinding into a powder and extracted several times with aqueous ethanol until decolorization of ethanol. The resultant solvent was evaporated by a rotary; the remaining part was partitioned amidst water and chloroform and left for overnight. The top aqueous layer has been collected and treated with n-butanol, after that, it was evaporated and concentrated in a drying oven [8].
Polysaccharides Preparation
Sea cucumber has been ground into powder; the powder has been mixed with distilled water in a conical flask. It extracted by using boiling distilled water and then filtered. Filtrates treated with trichloroacetic acid and left overnight to precipitate protein then its centrifuges, the filtrate was precipitated with four volumes of 95% (v/v) ethanol and left overnight at 4°C. The sediment which gained via cooling centrifuge at 4000 rpm for 10 minutes [27], and then the supernatant was waste. Also, both saponin and Polysaccharides were extracted during extract each other, briefly, sea cucumber has been ground into powder and after that it minced in boiling distal water for polysaccharides extraction and after the end method the ground sea cucumber has been put in aqueous ethanol for saponin extraction, but the yield is very limited, so the best methods of the extraction extract each of them alone.
Naringin, Hesperidin and Hesperitine Extraction
Both naringin and hesperdine have been extracted from mature citrus orange peels, Citrus sinensis (L.) Osbeck var. Balady (Rutaceae), was purchased from the Egyptian market and has been identified by prof. Kamal H. Shaltout and Dr. Thanaa M. A. EL-Komi (The Herbarium-TANE, Botany department, Faculty of Science, Tanta University, Egypt, Herbarium- TANE, Index Herbariorum New York Botanical Garden). Air dried citrus orange peels were ground into powder. Naringin has been extracted according to [28,29], with some modulation, 50 g of the husk powder has been added to aqueous ethanol. The flask was put into an ultrasonic bath with a frequency of 40 kHz (SB-120D, Xinzhi Technology, China) and kept for 2 hours and left overnight in the aqueous ethanol, then the orange liquid was filtered with Whatman filter paper, this procedure was repeated until decolonization of the ethanol. The filtrate has been concentrated by a rotary to remove the ethanol and obtain syrup consistency. Distilled water has been added to the obtained concentrated syrup, the mixture was agitated at 70 °C on a hot plate. 10 ml of methylene chloride has been added and the mixture left for 4 days at 25 °C to allow crystallization of naringin in the aqueous layer.
The naringin crystals were then collected by filtration. Hesperidin has been extracted according to the method described by Belboukhari et al. (2015) with some modification, 80 grams of the dried citrus orange peels was soaked in the petroleum ether after that heated to about 40°C for 1.5 h by using a hotplate, after filtration of the hot mixture through a Whatman filter paper no.1, the powder was allowed to dry at room temperature. After that, about 50 g of the new powder was extracted with 600 mL of ethanol, The extract was evaporated at rotary evaporator at 70oC for 30 min until syrup consistency was reached, the concentrated residual liquid was acidified to pH 3 with 6% acetic acid. The remaining part was preserved nocturnal in cold at 4oC. The precipitated solid was the crude hesperidin. The crude hesperidin was filtered with Whatman filter paper no.1 and washed with 6 % acetic acid. The obtained material was dissolved in dimethylformamide with continuous stirring and heating to approximately 60 °C. Then, the equivalent quantity of distilled water was added gradually, then it was cooled to precipitate the hesperidin and washed with little warm water.
Conversion of Hesperidin into Hesperitin:
A known weight of hesperidin which has been extracted from citrus orange husk has been added to methanol, then concentrated sulfuric acid has been added in a water bath, stirred and heated about 8 hours. The homogeneous solution which obtained was cooled, diluted by ethyl acetate and after that, it washed with distilled water. Hesperetin has been refined via dissolving in a small amount of acetone, and the resulting solution was added to a stirred mixture of distilled water and acetic acid. The gained hesperetin has been washed and cooled [30].
Identification of the Extracted Saponin, Naringin, Hesperidin and Hesperitin:
Fourier Transforms Infrared Spectroscopy (FT-IR): The functional groups of all extracted compounds, saponin, Polysaccharides, naringin, hesperidin and hesperetin were distinguished by Fourier transform infrared spectroscopy (Model- JASCO FT-IR4100 LE, made in Japan Range: 4000-400 cm-1) in the region of infrared radiation in the [Micro analytical unit, Faculty of Science, Tanta University, Egypt]. Briefly, 2 mg of saponin, Polysaccharides, naringin, hesperidin, and hesperitin were grounded and crushed to quite a powder with a mortar and pestle and was mixed with potassium bromide (KBr). Pellet which formed with the help of mechanical pressure was observed at the different coming wavelengths in FT-IR, infrared spectrum.
Determination of the Maximum Wavelength by Using Ultraviolet Spectroscopy (UV): Maximum wavelength of each component is unparalleled, and it can be utilized for specific definition the component. Saponin, Polysaccharides, naringin, hesperidin, and hesperetin extract were detected by Pg instruments (UV/vis spectrometer T80, Micro analytical unit, Faculty of Science, Tanta University, Egypt). Maximum wavelength of any component is known as the wavelength that the component displays the farthest absorbance. Triterpenoid marine sea cucumber saponin extract was soluble in distilled water. Meanwhile, naringin, hesperidin, and hesperitin were dissolved in dimethyl sulfoxide (DMSO), the prepared extract, solutions were measured for absorbance in UVVisible spectrophotometer in the UV region (200 nm-800 nm) and readings were noted down against blank. The graph was drawn between the obtained absorbance and wavelength. The peak obtained from the graph was taken as the most wavelength of that compound.
X-Ray Diffraction Analysis: X-Ray Diffractometry, the patterns of all extracted samples saponin, polysaccharide, naringin, hesperidin, and hesperitin were determined using the X-ray diffractometer (Siemens D5000, Germany). The investigated angularity has been adjusted from 2° ≤2θ≥ 50°, and the scanned rate average was 1°/min.
Thermal Gravimetric Analysis: Changes in the thermal properties of saponin, polysaccharide, naringin, hesperidin, and hesperitin were determined using [Schimadzu TG-50 thermogravimetric analyzer]. Briefly, the dried sample was placed in a previously tarred stainless-steel pan and weighted then heated from 25 °C to 800 °C at the rate of 10 °C /min under nitrogen supply of 10 mL/min (Micro analytical unit of faculty of science, Tanta University, Egypt).
Antioxidant Activity
Free Radical Scavenging of DPPH Radical: DPPH is an antioxidant method; depend on electron-transport which offers a violet solution in methanol [31]. Such free radical, constant at ambient temperature, it is reduced in the existence of an antioxidant compound, The reduction in the absorption and the changes in the color from dark violet to light violet or yellow color of the DPPH solution after the addition of an antioxidant has been read at 517nm., the utilize of the DPPH method supply a simple and quick technique to estimate antioxidants activities of the compounds under study by spectrophotometer [31]. The free radical scavenging actions (AA) of all compounds were studied according to [32] with some modifications. DPPH was prepared by weighing 0.025g and dissolved in methanol which gives absorbance 0.90 at 517 nm [33], 25μl of the extract was added to 975μl of methanolic DPPH, the methanolic DPPH extract mixture was shaken and put to stand in dark place and the absorbance was measured by JENWAY 6305 UV/ visible spectrophotometer at 517 nm. Ascorbic acid was utilized as a standard. The concentration of extract under study that can diminish by 50% (IC50) amount was calculated. The free radical scavenging action of the compound has been studied
% of Scavenging (AA) = (Abs of control −A
Abs of the sample) / Abs of control×100 .
Evaluation of Total Antioxidant Capacity: The total antioxidant capacity of the extracted compounds under study was measured as per Phosphomolybdate method according to the proposal of [34], Phosphomolybdate method is a spectroscopic technique that utilized to determine total antioxidant capacity, via formation of phosphomolybdenum compound. The principle of this assay depends on the reduction of Mo (VI) to Mo (V) by the examined extract and next formation of a blue-green phosphate Mo (V) complex at acidic medium [35], Briefly, 1 mL of phosphomolybdate was put in a glass tube and followed by 100 μL of the extract under study was added. The reaction was kept away from light and it incubated at 95 °C for 90 min. A blank without sample was also run. Subsequent the incubation time, the mixture has been cooled with tap water and measured by a spectrophotometer at 765 nm. ascorbic acid was measured as a reference.
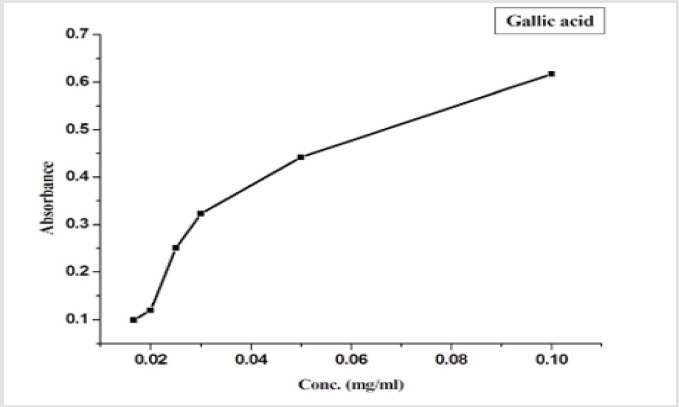
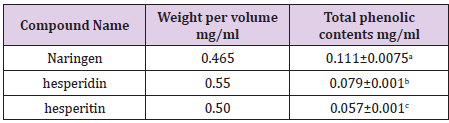
Determination of Total Phenolic Content: The Total Phenolic Content (TPC) has been measured according to [36], by using Folin Ciocalteu reagent, the principle methods depend on the reduction of tungsten and molybdenum oxides which arises a blue color, that can be measured at 750 nm by a spectrophotometer. The concentration of the extracted compounds under study was calculated from the standard curve of gallic acid (0.017 - 0.1 mg/ml).
Antibacterial Activity
Saponin, naringin, hesperidin and hesperetin extract were dissolved in distilled water and dimethyl sulfoxide respectively and their antibacterial activities were determined using the agar well method. Briefly, fifty μl of the extract under study was transferred into each hole in the test plate Petri dish. The appearance of a clear zone around the well in the inoculated plates is a sign of antibacterial activities of the strains under study (all in triplicate). The following test microorganisms were used for such purpose: Bacteria (prokaryotic): Gram-positive cocci: Streptococcus pyogenes, Staphylococcus aureus, and Gram-negative bacteria: Escherichia coli, Klebsiella pneumonia, and Proteus mirabilis.
Cytotoxicity Assay
Human hepatocellular carcinoma cells (HepG2) cells and Caco- 2 cells (colon cancer cell) have been seeded in 96-well plate at a density of 5×10, 1×10, 2×10, and 4×10 cells/well (in triplicate). The cells have been treated with the different compounds under the study at different concentration and measured after 22h, post-treatment by MTT assay. Absorbance in control and treated compounds wells have been detected by micro plate reader Elisa [37].
Determination of Cell Viability by Using MTT Assay
Principle: Cell viability has been studied by the usage of 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide dye (MTT) as the proposed method described by Oka [38]. The change of yellow color of MTT to purple color formazan which occurs only of the viable cells in the mitochondria that indicated the activity of reductase enzymes. The insoluble purple formazan product is dissolved in acidified isopropanol and the absorbance is measured at 630 nm using the microplate reader Elisa.
Statistical Analysis: The obtained data were statistically analyzed by one-way analysis of variance (ANOVA) followed by the Duncan multiple tests. All analyses were performed in triplicate and are expressed as average as mean values ±SEM using Co Stat 6.311. Values of P ≤ 0.05 were considered significant. Origin 6 application was used to be carried out statistical analysis and drawing the figures of results obtained.
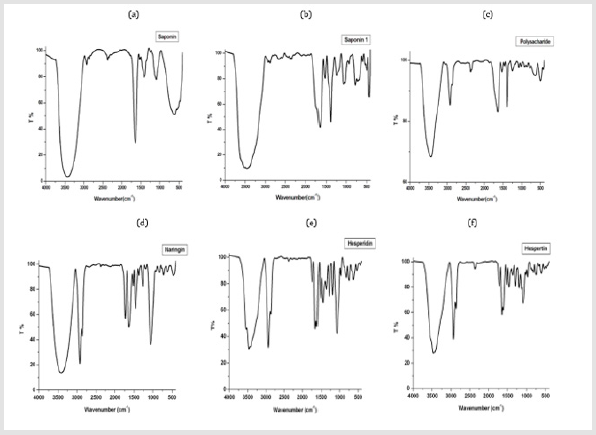
Figure 2: FT-IR spectrums of the saponin extract.
(a) First method of preparation and
(b) The second method,
(c) FT-IR spectrum of Polysaccharide extract,
(d) Naringin extract,
(e) Hesperidin and
(f) Hespertin.
Results and Discussion
Sea cucumbers characteristic by efficient foods, and it is a
good source of bioactive components [39], they’re worth because
of their content of valuable ingredients, for instance, saponin
and polysaccharide. The utmost plentiful flavonoids in citrus
fruit are hesperidin, narirutin, and neohesperidin; which can be
extracted by aqueous ethanol or methanol solutions [40]. Saponin,
polysaccharide, naringen, hesperidin, and hesperitin have been
extracted by successful methods as described and were identified
by several methods, for example, FT-IR of saponin (Figure 2),
confirmed the existence of saponins in the marine sea cucumber.
Saponins showed characteristic bands infrared absorbance of the
hydroxyl group (OH) in 3425 and 3470 cm-1. Carbon-hydrogen
(C=H) absorption ranged from 2927cm-1. The C=C absorbance
was observed at 1406 and 1535 cm-1. Whereas C=O absorbance
was found to be at 1639 and 1645 cm-1. Oligosaccharide linkage
absorptions to sapogenins, that is C-O-C, were evident in 1096 cm-1
region [8].
The aforementioned infrared functional group absorptions,
characteristic of saponins, have been referred to the existence of
the oleanolic acid-ester, which characterized by the C=O infrared
absorbance, these triterpenoid saponins are bidesmosides
because they own two connexions by glycones to the sapogenin
identified by glycosidic and ester groups. The meaning of these
results that saponins are detectable in both crude aqueous and /
or alcoholic extracts by using FT-IR spectroscopy [41]. Moreover,
the FT-IR spectra of the extracted polysaccharide are displayed in
(Figure 2) and exhibit the typical signals in the range from 4000
to 400 cm-1. The characteristic strong broad absorption at 3431.34
cm-1 corresponded to the O-H groups. The absorption peaks
at 2927.37 cm-1 and 2862.68 cm-1 indicated the aliphatic C–H
stretching vibrations. The strong band at 1639.62 cm-1 indicated
the absorption of C=O. The results of FT-IR spectroscopy could be
used to elucidate the configuration of the polysaccharide [42], a
band at 1465 and 1388 cm-1 were on behalf of the C-H deformation
vibration, the signals at 851–1245 cm-1 can be defined as fingerprint
area of carbohydrates, among which the bands at 1018, 1082 cm-1
were the characteristic absorptions of the pyranose ring.
Typical peaks at 928cm-1, 837 cm-1, 779 cm-1, was assigned to
the configuration of α-glycosides [43,44], characteristic absorption
bands at 1245 cm-1 (S=O stretching vibration) confirmed the
presence of sulfate groups, a band at 1070.96 suggest the presence
of C- O bonds [45]. The infrared spectrum of naringin in KBr
pellets showed characteristic bands, the OH group at 3411.61 cm-1,
the C-H at 2926.49 and 2861.55 cm-1[46], the band at 1729 cm-1
which indicated presence of C=O the carbonyl stretching vibration
of the carboxyl group (COO), The infrared bands around 1626
cm-1 which indicated the C=C stretching that is attributed to the
presence of aromatic or benzene rings. The vibrational bands at
around 1457.86 cm-1 were aliphatic and aromatic (C–H) group. The
bands in the range 1360–1050 cm-1 were due to the C–O stretching
vibration of carboxylic acids and alcohols and band at 1263 and
1180 cm-1 indication of C-O-C and O–H of polysaccharides. Spectra
absorbance at the wavenumber of 900 cm-1 or less was assigned
to be the fingerprint zone [47], and the peak absorbance at 1450
cm-1 and 1080 cm-1 is characteristic to the benzene ring stretching
vibrations [48].
Figure 3: UV- spectrum of
(a) Saponin (λmax 282 nm),
(b) Polysaccharide (λ max 262nm),
(c) Naringin (λmax 215, 296 and396 nm),
(d) Hesperidin (λmax 296, 312 nm) and
(e) Hespertine (λmax 296, 313 nm)
The FT-IR spectrum of hesperidin extract as KBr disk showed
a strong band of OH at 3554 and 3469 cm-1, CH (aliphatic) at 2926,
2855 cm-1, C=C (aromatic) at 1645, 1600 cm-1 and of C=O at 1735
cm-1, C-O at 1283 and 1069 cm-1. The hespertine compound which
has been obtained from hesperidin (Figure 2), The FT-IR spectrum
as KBr disk showed a strong band of OH at 3456.99 cm-1, CH
(aromatic) at 2926 – 2861 cm-1, C=C (aromatic) at 1638, 1600, 1515
cm-1 and of C=O at 1716 cm-1, C-O at 1186cm-1 and 1089 cm-1 [5].
Utmost of the saponins compounds display a major absorption peak
in the range of 250–350 nm. Saponin extracts have λ max at 282 nm
[8]. Furthermore, the λ max of sea cucumber polysaccharide has
been displayed in (Figure 3). UV-visible spectra are mostly utilized
for the testing chromophore groups of the atom that distinguished
by a strong absorbance electronic transition. The UV spectra in the
present research showed that the maximum absorbance was at 262
nm which is matching with [49].
Moreover, The UV spectrum of naringen extract showed
maximum absorption peaks at 215.2, 296 and 396 which in
agreement with [50] they showed the absorption peaks of naringen
at 214, 283.6 and 331.1nm. Furthermore, the UV spectrum of the
hesperdine extract showed maximum absorption at 296, 312,
and 345 nm. and for hespertine at 290 nm which in accordance
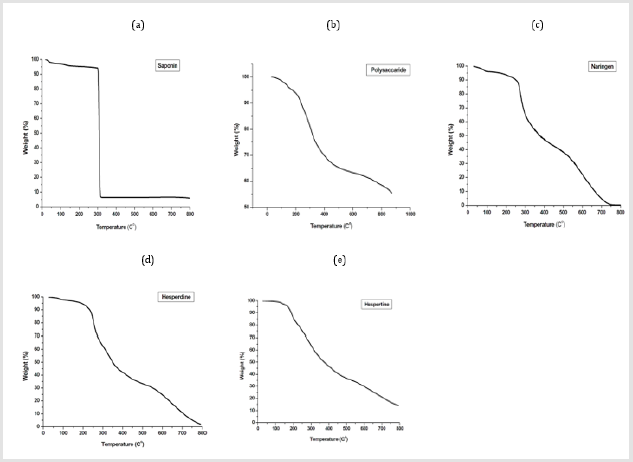
with [30]. The thermal behavior of the extracted compounds was
studied by TGA in the range of 25 to 800 °C be average 10 °C min−1
under a nitrogen atmosphere (Figure 5). TGA of sea cucumber
saponin, the thermal decomposition occurs in two successive
steps, which indicated that saponin is a stable compound. Thermal
stability is one of the most main physicochemical properties for the
applications of the polysaccharide. The TGA analysis of isolated sea
cucumber polysaccharide was carried out and the experimental
results showed that, the degradation temperature occurs in three
successive steps.
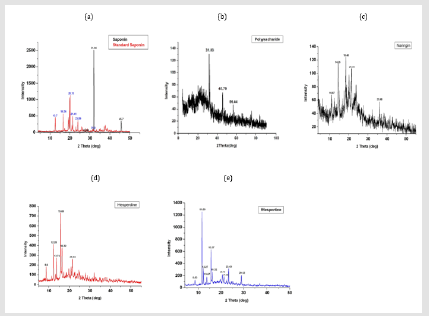
Figure 4: XRD of the
(a) extracted Saponin and commercial reference standard saponin,
(b) Polysaccharide,
(c) Naringin,
(d) Hesperidin and
(e) Hespertin.
The results indicated that marine sea cucumber polysaccharide
extract has altitude thermal steadiness. This data suggested that
marine sea cucumber polysaccharide extract could be used for
any function and in chemical adjustment [51] Such verity points
out that, the extract has not to be submitted to the degradation
temperature to not compromise the physical integrity of the extract.
TGA analysis of naringin showed that approximately 90% weight
loss has been observed more than 200 °C, the obtained data were
in agreement with [48,52], they confirmed the significant mass loss
which indicated the decomposition of naringen was found to be
around 250 °C. On the other hand, hesperdine decomposition steps
occur of about 63.74 % and 27.74 % has been identified between
266 and 500 °C and from 500 to 800 °C which can be attributed
to the decomposition of hesperidin Cao et al. (2018) and the
thermogram of hespetine extract show the thermal decomposition
of hesperetin occur of about 44.19 % and 19.9% which has been
identified between 241.4 and 550 °C and from 550 to 800°C.
The XRD analysis was performed to determine the crystalline
nature of the extracted compounds and to provide the qualitative
information of different elements in these compounds. The XRD
spectrum of both saponin sea cucumber extract and standard
saponin (Figure 4) displayed a distinct diffraction peak at 2θ
values of 31.94° and 45.7° and for standard at 12.7, 16.56,1
9.67, 20.13, 21.41, 23.89, 27.73, 31.86,37.63, 38.73 and 40.9°
respectively, which indicated that sea cucumber saponin is a highly
crystalline compound. On the other hand, the XRD of sea cucumber
polysaccharide has been displayed in (Figure 5), Showed a distinct
diffraction peaks at 2θ values of 31.83°, 45.79 and 56.44 which in
agreement with [42], polysaccharide being semi-crystal and did
not have periodical structure, could only show a diffuse region
corresponding to the maximum value of the diffraction when
the X-ray passed through the polysaccharide. It was difficult or
even unable to make a judgment on the chemical composition of
polysaccharide [42].
Figure 5: TGA analysis of
(a) Saponin,
(b) Polysaccharide,
(c) Naringen,
(d) Hesperidin and
(e) Hespertin.
Moreover, the X-ray of naringin showed that naringin was found to be a highly crystalline material as confirmed by various peaks in the diffractogram which in accordance to [52], there are five of the most prominent peaks from naringin diffractogram at angles of 10.87º, 14.26º, 18.48º, 21.31 and º35.98 were detected, confirming the presence of naringin in a crystalline form. The XRD results of hesperidin extract (Figures 6-9) there are six of the most prominent peaks from hesperidin diffractogram at angles of 8.6°, 12.28°, 13.72°, 15.69°, 16.32 and 21.53° were detected which confirmed that hesperdine existed in crystal form [53]. Also, the XRD of hesperetin showe that hespertine have 10 of the most prominent peaks from hesperetin diffractogram at angles of 8.63°, 11.69°, 12.27°, 13.67°, 15.57, 16.32, 20.70, 21.44, 23.43 and 29.12 which showed the crystalline form. Free radicals are thought to have a remarkable part in numerous diseases.
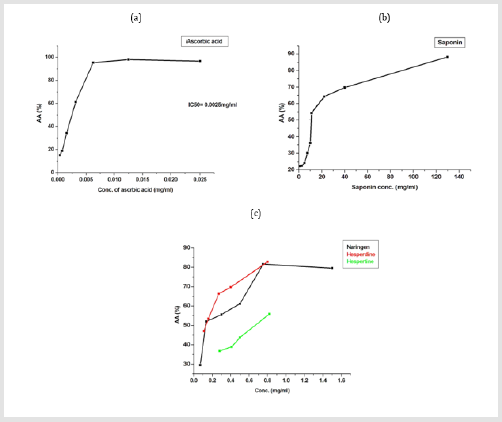
Figure 6: Percentage of DPPH radical scavenging activity of
(a) ascorbic acid,
(b) Saponin and
(c) Naringen, Hesperidin and hespertin.
Figure 7:
a) Total antioxidant activity of ascorbic acid,
(b) Saponin,
(c) Naringen, Hesperidin and hespertin
It should be studied to measure them and display the oxidative
damage that they cause [54]. Vitamin C is a potent antioxidant, that
can scavenge singlet oxygen, superoxide, and hydroxyl radicals
have a positive effect of a scavenger of free radicals [55]. The DPPH
method was utilized to detect the ability of the extract under study
in scavenging free radical [56]. In the DPPH radical scavenging
method, antioxidant compounds combine with DPPH and convert
it pale violet. The grade of change dark violet color to pale violet or
yellow denotes the ability of the compound to scavenge free radical
[57]. It has been shown that all extracted compounds under study
can effectively scavenge DPPH, the scavenging reaction between
DPPH and antioxidant compounds (H-A) is due to the capacity of
the extracted compounds to change DPPH color as a stable free
radical. For example, saponin extract IC50% was 10.50 mg/ml
which in agreement with [58].
Saponin may be depending on their structure in eliminating
free radical as it contains a number of the hydroxyl group (OH) in
its structure. DPPH is vastly utilized to estimate the free radical
scavenging of different antioxidant materials and polyhydroxy
aromatic components [59]. Naringen orange peels extract
which scavenged 50% of DPPH radical was 0.13 mg/ml which
in agreement with [60]. Also, Hesperidin extract showed potent
scavenging activates of free radical which scavenged 50% by 0.13
mg/ml. The antioxidant properties of hesperidin result from their
chemical structure, hydroxyl and methoxy system, the mutual
configuration of the double bond and the carbonyl group of the
C ring, and arrangement of the hydroxyl group and double bond
[61]. On the other hand, hespertine scavenged DPPH radical with
IC50 % equal to 0.66 mg/ml. Moreover, total antioxidant capacity is
determined through phosphomolybdenum complex formation [34]
to confirm its antioxidant properties.
The results also showed that the total antioxidant capacity of all compounds under the study increased with the increase of its concentration. The extracted compounds have the ability to scavenge free radicals and could act as a strong free radical inhibitor or scavenger due to its chemical structure and its ability to donate electrons, which is in accordance with [62]. The total phenolic content was determined using the folin ciocalteu reagent which gives a blue color to the solution; this indicated that orange peels have phenolic compounds which confirm the structure of extracted compounds from orange peels (Table 1). The hespertine extract showed a small clear zone around the well in the inoculated plates of Staphylococcus aureus which is an indication of antibacterial activities of the strains under this study this is may be due to the hesperidin hydrolysis and the change of their structure. MTT assay was determined to investigate the biological activity of all extracted compounds under study.
MTT assay showed that saponin, hesperidin, and hespertin on HepG2 showing higher affinities. IC50 represents the concentration that reduces cell viability by 50%. The mechanism of the extracted components as antioxidants may be due to inhibit the peroxidation of linoleic acid induced by Fe2+ and auto-oxidation in membranes cerebral and inhibiting the production of reactive oxygen species including hydroxyl radicals and nitric oxide [63] Saponins displayed anticancer properties by attaching various cancer-related proteins and Pathways [64]. So, marine-derived natural products such as saponins which represent a curative characteristic to fight cancer
Conclusion
The extracted compounds have been extracted and confirmed by FT-IR, UV, XRD and TGA analysis. The obtained data indicated that sea cucumber saponin, polysaccharides, and orange peels, Naringin, Hesperidin, and Hesperitin may provide a promising new therapeutic approach to HEPG2 cancer cells. In addition, these compounds were an effective antioxidant, so they may be effective and scavenge free radicals which resulted from the disease and may manage corona virus.
Acknowledgement
We wish to express our sincere thanks and deepest to Prof. Dr. Nadia A. El-Wakiel Professor of inorganic chemistry, faculty of Science Tanta University, for her continuous help and cooperation in the interpretation of the TGA and to Dr. Thanaa Mahmoud Ali EL-Komi (Ecology, Herbarium –TANE, faculty of Science Tanta University) for her continuous help and cooperation.
Conflict of Interests
The author declares that there is no conflict of interest.
References
- Assefa AD, Saini RK, Keum YS (2017) Extraction of antioxidants and flavonoids from yuzu (Citrus junos Sieb ex Tanaka) peels: a response surface methodology study. J Food Meas Charact 11: 364-379.
- Vandavasi SR, Ramaiah M, Gopal PN (2015) In vitro standardization of flowers of methanolic extract of Dendrobium normalefalc. For free radical scavenging activity. Journal of Pharmacognosy and Phytochemistry 3(5): 107-111.
- Lou Y, Huang G, Zhao Y, Lu X, Chen JC, (2013) Protective role of the polysaccharides from sea cucumber, Acaudina molpadioidea, in cecal ligation and puncture-induced sepsis. CURRENT topics in nutraceutical research 11(1-2): 29-34.
- Qi H, Jia X, Liu S, Feng D, Dong X, et al. (2017) Antioxidant and anti-dyslipidemic effects of polysaccharidic extract from sea cucumber processing liquor. Electronic Journal of Biotechnology 28: 1-6.
- El Barky AR, Ali EMM, Mohamed TM (2017) Marine Sea Cucumber Saponins and Diabetes. Austin Pancreat Disord 1(1): 1-7.
- Zhu BW, Zhou DY, Li T, Yan S, Yang JF, et al. (2010) Chemical composition and free radical scavenging activities of a sulphated polysaccharide extracted from abalone gonad (Haliotis Discus HannaiIno). Food Chem 121: 712-718.
- Guo M, Song F, Liu Z (2006) Characterization of triterpenoidic saponin -mixturein crude extracts from leaves of Acanthopanax senticosus harms by saponin structural correlation and mass spectrometry. Analytica Chimica Acta 57(1-2): 198-203.
- El Barky AR, Hussein SA, Alm Eldeen AA, Hafez YA, Tarek M, et al. (2016) Anti-diabetic activity of Holothuria thomasi Biomedicine & Pharmacotherapy 84: 1472-1487.
- Li G, Kim DH, Kim TD, Park BJ, Park HD, et al. (2003) Protein-bound polysaccharide from Phellinuslinteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett 216(2): 175-181.
- Suetsugu T, Iwai H, Tanaka M, Hoshino M, Quitain A, et al. (2013) Extraction of Citrus Flavonoids from Peel of Citrus Junos Using Supercritical Carbon Dioxide with Polar Solvent. Chemical Engineering and Science 1(4): 87-90.
- Benavente Garcia O, Castillo J (2008) Update on uses and properties of citrus flavonoids: new findings in anticancer, cardiovascular and anti-inflammatory activity. Journal of Agricultural and Food Chemistry 56: 6185-6205.
- Izquierdo L, Sendra JM (2003) Citrus Fruits Composition and Characterization. In: Encyclopedia of Food Sciences and Nutrition. B Caballero, L Trugo, P Finglas(Eds.) Oxford: Oxford Academic Press, 6000.
- Peterson JJ, Dwyer JT, Beecher GR, Bhagwat SA, Gebhardt SE, et al. (2006) Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: a compilation and review of the data from the analytical literature. J Food Compost Anal 19: S66-S73.
- Gorinstein S, Huang D, Leontowicz H, Leontowicz M, Yamamoto K, et al. (2006) Determination of naringin and hesperidin in citrus fruit by high-performance liquid chromatography. The antioxidant potential of citrus fruit. acta chromatographica 17: 108.
- Horcajada MN, Habauzit V, Trzeciakiewicz A, Morand C, Gil Izquierdo A, et al. (1985)Hesperidin inhibits ovariectomized-induced osteopenia and shows differential effects on bone mass and strength in young and adult intact rats. J Appl Physiol 104(3): 648-654.
- Meneguzzo F, Ciriminna R, Zabini F, Pagliaro M (2020) Accelerated production of hesperidin-rich citrus pectin from waste citrus peel for prevention and therapy of COVID-19. Preprints.
- Chen YW, Yiu CPB, Wong KY (2020) Prediction of the SARS-CoV-2 (2019-nCoV) 3C-like protease (3CLpro) structure: virtual screening reveals velpatasvir, ledipasvir, and other drug repurposing candidates. F1000Research 9: 129.
- Pękal A, Dróżdż P, Biesaga M, Pyrzynska K (2011) Evaluation of the antioxidant properties of fruit and flavoured black teas. Eur J Nutr 50(8): 681-688.
- Jain M, Parmar HS (2011)Evaluation of antioxidative and anti-inflammatory potential of hesperidin and naringin on the rat air pouch model of inflammation. Inflamm Res 60(5): 483-491.
- Nie YC, Wu H, Li PB, Luo YL, Long K, et al. (2012) Anti-inflammatory effects of naringin in chronic pulmonary neutrophilic inflammation in cigarette smoke-exposed rats. J Med Food 15(10): 894-900.
- Choi EJ, Lee JI, Kim GH (2011) Effects of 4′, 7-dimethoxyflavanone on cell cycle arrest and apoptosis in human breast cancer MCF-7 cells. Arch Pharm Res 34(12): 2125-2130.
- Itoh K, Masuda M, Naruto S, Murata K, Matsuda H (2009) Antiallergic activity of unripe citrus hassaku fruits extract and its flavanone glycosides on chemical substanceinduced dermatitis in mice. J Nat Med 63(4): 443-450.
- Badea M, Olar R, Uivarosi V, Marinesu D, Aldea V (2012) Synthesis and characterization of some vanadyl complexes with flavonoid derivatives as potential insulin-mimetic agents. J Therm Anal Calorim 107: 279-285.
- Rong W, Wang J, Liu X,Jiang L, Wei F, et al. (2012) Naringin treatment improves functional recovery by Increasing BDNF and VEGF expression, inhibiting neuronal apoptosis after spinal cord injury. Neurochem Res 37(8): 1615-1623.
- Yu X, Zhao X, Wu T, Zhou Z, Gao Y, et al. (2013) Inhibiting wear particles induced osteolysis with naringin. Int Orthop 37(1): 137-143.
- Hu X, Wang Y, Wang J,Xue Y, et al. (2010) Dietary saponins of sea cucumber alleviate orotic acid-induced fatty liver in rats via PPARa and SREBP-1c signalling. Lipids Health Dis 9: 25.
- Wu YC, Liang ZC, Lu CP, Wu SH (2008) Effect of Carbon and Nitrogen Sources on the Production and Carbohydrate Composition of Exopolysaccharide by Submerged Culture of Pleurotus citrinopileatus. Food and Dr Analysis16: 61-67.
- Sudto K, Pornpakakul S, Wanichwecharungruang S (2009) An efficient method for the large scale isolation of naringin from pomelo (Citrus grandis) peel. International Journal of Food Science and Technology 44: 1737-1742.
- Tang D, Zhu C, Zhong S, Zhou MD (2011) Extraction of naringin from pomelo peels as dihydrochalcone’s precursor. J Sep Sci 34(1): 113-117.
- Belboukhari NLN, Cheriti A, Sekkoum K (2015) Hesperidin and hesperitin preparation and purification from Citrus sinensis Der Pharma Chemica 7(2): 1-4.
- Huang DJ, Ou BX, LPrior R (2005) The chemistry behind antioxidant capacity assays. J Agric Food Chem 53(6): 1841-1856.
- Wang M, Shao Y, Li J, Zhu N, Rangarajan M, et al. (1998) Antioxidative phenolic compounds from sage (Salvia officinalis). J Agric Food Chem 46(12): 4869-4873.
- Zengin G, Aktumsek A, Guler GO, Cakmak YS, Yildiztugay E (2011) Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei Subsp Hayekiana Wagenitz Rec Nat Prod 52: 123-132.
- Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of Vitamin E1. Anal Biochem 269(2): 337-341.
- Singh S, Singh RP (2008) In vitro methods of assay of antioxidants: An overview. Food reviews international 24(4): 392-415.
- Komal F, Haroon R, Jelani S, Masood H (2015) A relative in vitro evaluation of antioxidant potential profile of extracts from pits of Phoenix dactyliferaL. (Ajwa and Zahedi Dates). Int J Adv Sci Technol 35(35): 2319-2682.
- Teng BS, Lu YH, Wang ZT, Tao XY, Wei DZ (2006) In vitro anti-tumor activity of isorhamnetin isolated from Hippophae rhamnoides L. against BEL-7402 cells.Pharmacol Res 54(3): 186-194.
- Oka M, Maeda S, Koga N, Kato K, Saito T (1992) A modified colorimetric MTT assay adapted for primary cultured hepatocytes: application to proliferation and cytotoxic assays. Bioscience Biotechnology and Biochemistry 56(9): 1472-1473.
- Qi H, Fu H, Dong XF, Feng D, Li N, et al. (2016) Apoptosis induction is involved in UVA-induced autolysis in sea cucumber Stichopus japonicus. J Photochem Photobiol B 158: 130-135.
- Ma Y, Ye X, Fang Z, Chen JC, Xu GH, et al. (2008) Phenolic Compounds and Antioxidant Activity of Extracts from Ultrasonic Treatment of Satsuma Mandarin (Citrus unshiu Marc.) Journal of Agricultural and Food Chemistry 56(14): 5682-5690.
- Almutairi MS, Ali M (2014) Direct detection of saponins in crude extracts of soapnuts by FTIR. Natural Product Research 29(13): 1271-1275.
- Zhao S, Li B, Chen G, Hu Q, Zhao L (2017) Preparation, characterization, and anti-inflammatory effect of the chelate of Flammulina velutipes polysaccharide with Zn. Food and agricultural immunology 28(1): 162-177.
- Miao M, Ma Y, Jiang B, Huang C, Li X, et al. (2014) Structural investigation of a neutral extracellular glucan from Lactobacillus reuteri Carbohydrate Polymers 106: 384-392.
- Liu W, Wang H, Yu J, Liu Y, Lu W, et al. (2016) Structure, chain conformation, and immunomodulatory activity of the polysaccharide purified from Bacillus Calmette Guerin formulation. Carbohydrate Polymers 150: 149-158.
- Yang X, Wang R, Zhang S, Zhu W, Tang J, et al. (2014) Polysaccharides from Panax japonicus CA Meyer and their antioxidant activities. Carbohydr Polym 101: 386-391.
- Diaz Uribe CE, Vallejo W, Oliveros G, Amner Muñoz A (2016) Study of scavenging capacity of naringin extracted from Citrus uranium peel against free radicals. Prospect 14(2): 31-35.
- Ernawita Wahyuono RA, Hesse J, Hipler UC, Elsner P, Böhm V (2017) In Vitro Lipophilic Antioxidant Capacity, Antidiabetic and Antibacterial Activity of Citrus Fruits Extracts from Aceh, Indonesia. Antioxidants 6(1): 11.
- Feng X, Wu T, Yu B, Wang Y, Zhong Shian Z (2017) Hydrophilic surface molecularly imprinted naringin prepared via reverse atom transfer radical polymerization with excellent recognition ability in a pure aqueous phase. RSC Adv 7: 28082-2809.
- Trabelsi L, M’sakni N, Ouada HB, Bacha H, Roudesli S (2009) Partial Characterization of Extracellular Polysaccharides Produced by Cyanobacterium Arthrospira platensis. Biotechnology and Bioprocess Engineering 14: 27-31.
- Sun Y, Wang J, Gu S, Liu Z, Zhang Y, et al. (2010) Simultaneous Determination of Flavonoids in Different Parts of Citrus reticulata ‘Chachi’ Fruit by High Performance Liquid Chromatography-Photodiode Array Detection. Molecules 15(8): 5378-5388.
- Xu C, Yang C, Mao D (2014) Fraction and chemical analysis of antioxidant active polysaccharide isolated from flue-cured tobacco leaves. Pharmacogn Mag 10(37): 66-69.
- Pai DA, Vangala VR, Ng JW, Tan RBH (2015) Resistant maltodextrin as a shell material for encapsulation of naringin: Production and physicochemical characterization. Journal of Food Engineering 161: 68-74.
- Varghese JJ, Mallya R (2015) Formulation development and evaluation of antioxidant potential of hesperidin nanocrystals. World Journal of Pharmaceutical Research 4(08): 1149-1170.
- Halliwell B, Whiteman M (2004) Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean. Br J Pharmacol 142(2): 231-255.
- Das KK, Das SM, Dasgupta S (2001) The influence of ascorbic acid on nickel induced hepatic lipid peroxidation in rats. J Basic Clin Physiol Pharmacol 12(3): 187-195.
- Alothman M, Bhat R, Karim AA (2009) Antioxidant Capacity and Phenolic Content of Selected Tropical Fruits from Malaysia, Extracted with Different Solvents. Food Chemistry 115(3): 785-788.
- Lu Y, Khoo TJ, Wiart C (2014) Antioxidant Activity Determination of Citronellal and Crude Extracts of Cymbopogon citratus by 3 Different Methods. Pharmacology & Pharmacy 5(4): 395-400.
- MO N, AOT A (2017) Antioxidant and Inhibitory Effects of Saponin Extracts from Dianthus basuticus Burtt Davy on Key Enzymes Implicated in Type 2 Diabetes In vitro. Pharmacogn Mag 13(52): 576-582.
- Nishizawa M, Kohno M, Nishimura M, Kitagawa A, Niwano Y (2005) Non-reductive scavenging of 1, 1-diphenyl-2-picrylhydrazyl (DPPH) by peroxyradical: a useful method for quantitative analysis of peroxyradical. Chem Pharm Bull 53(6): 714-716.
- Pari L, Amudha K (2011) Antioxidant effect of naringin on nickel-induced toxicity in rats: an in vivo and in vitro International journal of pharmaceutical sciences and research. IJPSR 2(1): 137-144.
- Piskula MK (2000) Soy isoflavone conjugation differs in fed and food deprived rats. J Nutr 130(7): 1766-1771.
- Mishra K (2013) Structure-Activity Relationship of Antioxidative Property of Hesperidin. Int J Chem Stud 1(4): 2321-4902.
- Kim JY, Jung KJ, Choi JS, Chung HY(2004) Hesperetin: a potent antioxidant against peroxynitrite. Free Radic Res 38(7): 761-769.
- Xu X, Li T, Fong C, Chen X, Chen XJ, et al. (2016) Saponins from Chinese Medicines as Anticancer Agents. Molecules 21(10): 1326.

 Research Article
Research Article