Abstract
Aedes aegypti (L.) and Ae. albopictus (Skuse) are accepted as primary vectors of mosquito-borne diseases, such as Dengue Haemorrhagic Fever (DHF), chikungunya fever and Zika fever. As there is no effective vaccine to prevent these diseases, mosquito management, including chemical, biological and physical strategies, are the main practices used to control them. A novel lethal ovitrap, called the LeO-Trap®, was then developed as an additional device for part of the integrated control against Ae. aegypti and Ae. albopictus mosquitoes. It was created from combination of a physically attractive design of the ovitrap, with the biochemical attractant derived from carpet shell extract and AZAI: the larvicide formulated from zeolite granules containing 1% temephos. Gravid female Ae. aegypti and Ae. albopictus in the laboratory preferred to lay their eggs in the newly designed LeO-Trap® rather than the common ovitrap because the former and latter trap had physical attractiveness of 1.9 and 2.5 times, respectively. The oviposition efficacy of the LeO-Trap® against both species, Ae. aegypti and Ae. albopictus, also was increased dramatically at around 1.5 and 2.7 times, respectively, when combined with the attractant formulated from carpet shell extract. Finally, the LeO-Trap® with carpet shell attractant and AZAI killed all of the larvae that emerged from the eggs laid inside the trap. In field experiments, the LeO-Trap® collected many eggs of Ae. aegypti in urban houses and those of Ae. albopictus in a rubber plantation, and the abundance of both female species was reduced in the study areas within 12 weeks. In conclusion, the results obtained from this study revealed that the LeO-Trap® showed excellent efficacy in luring gravid female Ae. aegypti and Ae. albopictus into laying their eggs inside the trap, and all of the larvae that hatched from the eggs were killed eventually by AZAI. Therefore, the LeO-Trap® can be used as an additional tool in the integrated vector control program for controlling Aedes-borne diseases in Thailand and elsewhere.
Keywords: Lethal ovitrap; Aedes aegypti; Aedes albopictus; Dengue Haemorrhagic Fever; Zika; Chikungunya
Introduction
Mosquitoes can transmit pathogens that cause lethal diseases, such as malaria, Yellow Fever (YF), Dengue Haemorrhagic Fever (DHF), lymphatic filariasis and Japanese Encephalitis (JE) to humans [1]. Aedes aegypti (L.) and Ae. albopictus (Skuse) are recognized as principle mosquito vectors of dengue, yellow fever, chikungunya and Zika viruses, and many other arboviruses [2].
These diseases are major public health problems in many countries in tropical and sub-tropical regions. There is no effective vaccine available for the control of these diseases, except for yellow fever and JE. Mosquito control, therefore, is considered primarily as one of the main approaches to manage mosquito-borne diseases. The strategies used to control Aedes mosquitoes include elimination of mosquito breeding places, use of biological organisms (copepods, guppy fish) [3], application of larvicides containing various kinds of active ingredients, such as organophosphate (temephos) [4], biological agent (Saccharopolyspora spinosa) [5] and insect growth regulator (diflubenzuron) [6], adulticide application (etofenprox, cypermethrin) [7], and lethal ovitrap [8].
However, the strategies currently available are inadequate and limited in some factors [8,9]. At present, most vector control programs depend mainly upon the use of chemical insecticides and primary application of adulticides by using thermal fogging. However, insecticide resistance, especially against pyrethroids, has become a common occurrence in various vector mosquitoes, including Ae. aegypti and Ae. albopictus [10]. The use of insecticides has been hindered also by a growing concern about possible adverse effects on human health, the environment and nontarget organisms. Therefore, seeking devices that use the lowest application of insecticide instead of mass application for controlling Aedes mosquitoes is an important concept. Consequently, the ovitrap has been used for surveillance or monitoring Ae. aegypti and Ae. albopictus in endemic areas. Furthermore, it can be developed as a lethal ovitrap for controlling Aedes larvae and adults when incorporated with larvicides or adulticides. Many studies of lethal ovitraps have been carried out to control Ae. aegypti and Ae. albopictus populations in laboratories [11,12], semi-field [13] and the field [14-18].
This study aimed to evaluate the effectiveness of the newly developed lethal ovitrap from the combination of a physically attractive design with biochemical attractant and larvicide (LeOTrap ®) for controlling Ae. aegypti and Ae. albopictus. The newly developed lethal ovitrap obtained from this study can be used as an additional tool in the integrated vector control program for controlling Aedes-borne diseases in Thailand and elsewhere.
Materials and Methods
The Design of the Physically Attractive Ovitrap for Aedes Mosquitoes
A black plastic cup (8.5 cm in height, 7.5 cm in diameter at the bottom and 9.5 cm in diameter at the top) was a common ovitrap used for the oviposition of Ae. aegypti and Ae. albopictus in the laboratory or for surveillance in the field. The idea was to modify the common ovitrap to be an additional, effective tool for Aedes mosquito control in the field. Physical appearance of the ovitrap could be a factor in influencing Aedes mosquito oviposition. A new model of ovitrap was then designed and developed (LeO-Trap-V1), with improved attraction as a mosquito oviposition site. The body of the LeO-Trap-V1 was made from High Density Polyethylene (HDPE) with black color, 13 cm in height, 10 cm in diameter at the bottom and 7 cm in diameter at the top. It was round-shaped, similar to the water storage jars used commonly in Thailand. There was one hole for drainage (1.5 cm in diameter) on the body of the trap (9 cm from the bottom).
In addition, it was covered with a round roof of 12 cm in diameter and 4 cm in height to protect from rainwater and sunlight when being set up outdoors. This round roof was made from Polypropylene (PP). There were 8 windows (2 x 2 cm) between the body and roof, when they were attached together. These windows were designed for the mosquitoes to enter and fly inside the trap and lay their eggs. Both traps (Figure 1) were compared for the oviposition of Ae. aegypti and Ae. albopictus under laboratory conditions, with 10 replications (the details are described in the section “Oviposition experiments in the laboratory”).
Figure 1: Comparison of the commonly used ovitrap (a black plastic cup) on the left and the newly developed ovitrap (LeO-Trap-V1) on the right.
Development of a more Physically Attractive Ovitrap for Aedes mosquitoes
In developing a more physically attractive ovitrap, the LeOTrap- V1 was modified to increase efficacy against Aedes mosquito oviposition. It was observed that the entrances for mosquitoes to fly inside the LeO-Trap-V1 were small, so the modified LeO-Trap-V2 had a higher roof (6.5 cm) and bigger entrances (2 x 4.5 cm), while no alteration was made to the body of the trap. Both models (Figure 2) were compared for the oviposition of Ae. aegypti and Ae. albopictus under laboratory conditions, with 10 replications (the details are described in the section “Oviposition experiments in the laboratory”).
Figure 2: Comparison of the newly developed ovitrap (LeO-Trap-V1) on the left and the modified ovitrap (LeOTrap- V2) on the right.
Development of the Ovitrap with Attractant for Aedes Mosquitoes
The attractant used in this study originated from carpet shell extract [19]. Fresh carpet shells were extracted with water, and the extract was separated from the sediment by filtering. The carpet shell extract was formulated with polymers and stabilizing agents, and then prepared as microcapsule emulsion. The process of extraction and formulation of the carpet shell extract could not be described here in detail, as they are trade secrets and have been registered for Petty Patent at the Department of Intellectual Property, Ministry of Commerce, with the proprietary right between the Department of Medical Sciences, Ministry of Public Health, Thailand and Ikari Trading (Thailand) Co., Ltd. The LeO-Trap-V2 was selected for testing efficacy comparison of the attractant. The microcapsule emulsion of the carpet shell attractant was coated around the inside of the trap above the drain hole and also under the roof. In each experiment, two LeO-Trap-V2s (with and without attractant) were compared for number of eggs laid on the filter papers. The experiments were carried out against Ae. aegypti and Ae. albopictus under laboratory conditions, with 10 replications (the details are described in the section, “Oviposition experiments in the laboratory”).
Development of the Lethal Ovitrap for Aedes mosquitoes
The lethal ovitrap was developed finally from a combination of the LeO-Trap-V2 with carpet shell extract as an attractant and larvicide. The larvicide used in this study was zeolite granules containing temephos (1% w/w) as an active ingredient, called AZAI®. In each experiment, two LeO-Trap-V2s (both coated with attractant) were compared for larvicidal efficacy, when one was treated with AZAI® (approximately ¼ teaspoon) and the other one not. After removal of the traps from mosquito cages, the filter paper in each trap was left in the trap to observe larval hatching for 7 days and then numbers of larvae were count and recorded. The experiments were carried out against Ae. aegypti and Ae. albopictus under laboratory conditions, with 10 replications (the details are described in the section, “Oviposition experiments in the laboratory”).
Mosquitoes for Ovipositional Experiments in the Laboratory
The mosquitoes used in this study were laboratory-reared female Ae. aegypti and Ae. albopictus. They were cultured in the insectary according to the standard operational procedures of the National Institute of Health, Department of Medical Sciences, Thailand, and maintained in the insectary at temperatures of around 24-28oC, relative humidity of 60-80% and a 12L:12D photoperiod. The procedure for handling Ae. albopictus mosquitoes was similar to that for Ae. aegypti. In order to hatch mosquito eggs, those attached to filter paper were dipped into a plastic tray (26x36x6 cm) containing 2 liters of tap water and left for a few hours until all of the larvae had hatched. After hatching, the larvae were fed with mouse food ground into fine powder at about 200-300 mg per tray twice daily. Four larval instars were followed by the pupal stage, which lasted for a total of 6-10 days. Up until then, approximately 300-400 pupae were transferred from the tray to a plastic cup containing around 500 ml of tap water. The cup was placed in a mosquito cage (40x40x40 cm), where the adults emerged within a few days. Syrup containing 10% (w/v) sugar, prepared in a glass bottle with a cotton stick, was available at all times to the adults for sugar feeding in the cage.
Two days after emergence, the female mosquitoes were provided with artificial feeding apparatus, using recently expired human blood received from the Blood Bank of the Thai Red Cross. This blood had been approved as pathogen free, according to the standard procedures of the blood bank for blood collecting. The blood feeding was carried out for a few hours, and the apparatus was removed from the cage. A few days after the blood meal, the female mosquitoes became gravid and ready for egg laying.
Oviposition Experiments in the Laboratory
Fifty gravid females of Ae. aegypti or Ae. albopictus (aged 4-6 d, and 2-3 d after blood feeding) were released into the mosquito cage (40x40x40 cm), where traps for testing each experiment were located 15 cm apart. A piece of filter paper (Whatman no. 1, 9x32 cm) was attached inside each trap as a substrate for Aedes mosquito egg laying. Tap water was poured into the trap until its level reached approximately halfway up the filter paper. A piece of cotton stick soaked with syrup (10% w/v sugar) was put into a glass bottle and placed inside the cage to provide food for the mosquitoes. The cage was kept for 4 days in an environmentally controlled room with a photoperiod of 12L:12D, relative humidity of 60-80%, and temperature of 24-28°C. The numbers of eggs deposited on the filter paper in each trap were counted under a stereomicroscope and recorded. In studying the experiment of lethal effect, the filter papers containing the mosquito eggs were left in the traps of both groups (with and without AZAI) for hatching observation. Seven days after removal of the traps from mosquito cages, the numbers of larvae hatched from each filter paper of both groups were counted and recorded.
Evaluations of Oviposition Efficacy of the LeO-Trap® against Aedes Msosquitoes in the Field
The lethal LeO-Trap® was derived from a combination of the LeO-Trap-V2 with carpet shell attractant and AZAI® and evaluated for oviposition efficacy against Ae. aegypti and Ae. albopictus in the field. The lethal LeO-Trap® was set up for Ae. aegypti mosquitoes in 30 houses in a village in Muang district, Nonthaburi province, Thailand. One LeO-Trap® was placed inside each house near appropriate locations, such as a sofa in the living room, under the stairs, among clothes hangers, etc., while others were located outside the house in places such as shoes cupboards, flowerpots, tables, etc. A piece of white filter paper (9x32 cm.) was put inside the traps as a substrate for Ae. aegypti egg laying, and tap water was poured into the traps until the level reached about halfway up the filter paper. About ¼ teaspoon of AZAI® (approximately 1 gram) was put into the traps, before they were covered with the roof and placed in appropriate locations inside and outside the house. Baseline data at the beginning of the experiment were collected 4 days after the traps were set up.
The traps in each house were inspected, the filter papers replaced, and the water was topped-up to the appropriate level of about halfway up the filter paper. The filter papers with the mosquito eggs attached were brought back to the laboratory for egg counting. After that, assessments were carried out every 2 weeks for 3 months. Evaluations of adult Ae. aegypti populations were carried out in the experimental houses at the beginning of the experiment and then every 2 weeks, when the traps were examined for eggs. These adult populations were assessed by 2 volunteers, who used a sweep net for 10 minutes to collect the mosquitoes in each house, and all of the captured mosquitoes were identified by species and sex, while female Ae. aegypti were counted and recorded. The experiment against Ae. aegypti in the field was carried out for 12 weeks. On the other hand, it is recognized that Ae. albopictus mosquitoes in Thailand are always abundant in rubber and palm plantations and fruit orchards. Therefore, the field experiment against Ae. albopictus was carried out in a rubber plantation in Bang Lamung district, Chonburi province, Thailand. A total of 200 LeO-Traps® were placed in an area of about 7 Rai (approximately 11,200 m2). A piece of white filter paper (9x32 cm.) was put inside the traps as substrate for Ae. albopictus egg laying, with tap water poured into the traps until its level reached about halfway up the filter paper. Approximately 1 gram of AZAI® was put into the traps before they were covered with the roof, and each trap was hung on a rubber tree about 1 meter above the ground and 10 meters apart. Similar to the experiment against Ae. aegypti, the baseline data at the beginning of this study were collected 4 days after the traps were placed in the field. The traps were examined, filter papers replaced, and the tap water was topped-up to about halfway up the filter paper. The mosquito eggs attached to the filter papers were brought back to the laboratory for egg counting.
Then, assessments were carried out every 2 weeks for 3 months. Assessments of adult Ae. albopictus populations in the experimental area were carried out at the beginning of the experiment and then every 2 weeks, when the traps were inspected for eggs. These adult populations were assessed by 6 volunteers, who used a sweep net for 10 minutes to collect the mosquitoes, and all of those captured were identified by species and sex, while female Ae. albopictus were counted and recorded.
Data Analysis
The numbers of Ae. aegypti and Ae. albopictus eggs obtained from various experiments were compared for mean numbers (+ S.E.), and the t-test analysis was used for comparisons. The accepted level of significance for all comparisons was P<0.05. Analysis was carried out using the SPSS program for Windows (IBM SPSS Statistics 22.0).
Results
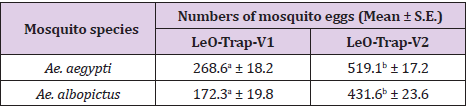
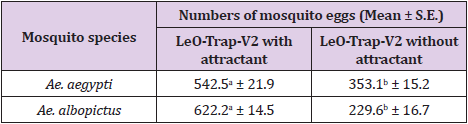
The mean numbers (+S.E.) of eggs laid by the gravid female Ae. aegypti and Ae. albopictus in the common ovitrap (black plastic cup) and newly developed ovitrap (LeO-Trap-V1) are shown in Table 1. Overall, the gravid females of both species preferred to lay their eggs in the LeO-Trap-V1 rather than the black plastic cup. The mean number of eggs for Ae. aegypti found in the LeO-Trap-V1 (519.1) was significantly higher than that in the black plastic cup (268.6) by about 1.9 times. A similar result was obtained from the gravid female Ae. albopictus, with the mean number of eggs laid in the LeO-Trap-V1 (431.6) being significantly higher than that obtained from the black plastic cup (172.3) by approximately 2.5 times. Although the newly developed ovitrap (LeO-Trap-V1) showed excellent efficacy against the oviposition of Ae. aegypti and Ae. albopictus, when compared with the commonly used ovitrap (the black plastic cup), it was found that the LeO-Trap-V1 could be developed further for improving results, as its entrances for the mosquitoes were inadequate. Therefore, the LeO-Trap-V1 was modified into the newly developed LeO-Trap-V2 and the comparison of efficacy is presented in Table 2. The modified LeO-Trap-V2 collected a significantly higher mean number of Ae. aegypti eggs (550.9) than the LeO-Trap-V1(319.4) by about 1.7 times. Similarly, the mean number of Ae. albopictus eggs obtained from the LeO-Trap-V2 (540.2) also was significantly higher than that from the LeO-Trap-V1(283.4) at around 1.9 times. As can be seen, the modification of bigger entrances for mosquitoes substantially influenced oviposition efficacy of the physically modified trap. The results in Table 3 reveal that the attractant derived from carpet shell extract effectively lured the gravid female Ae. aegypti and Ae. albopictus into laying more of their eggs in the ovitrap with it. The mean number of Ae. aegypti eggs laid in the LeO-Trap-V2 with attractant (542.5) was significantly higher than that in the LeO-Trap-V2 without it (353.1) by approximately 1.5 times. In the meantime, the LeO-Trap-V2 with attractant also collected significantly more Ae. albopictus eggs (622.2) than that without it (229.6) by about 2.7 times. The results obtained from this study revealed the significant efficacy of the attractant derived from the carpet shell extract against the oviposition of both Aedes species in Thailand.
Table 1: Mean number (+ S.E.) of eggs compared between those laid in the black plastic cup and those laid in the LeO-Trap-V1 by gravid female Ae. aegypti and Ae. albopictus.
Remark: The mean number of eggs in the same row followed by a different letter is significantly different (P < 0.001).
Table 2: Mean number (+ S.E.) of eggs compared between those laid in the newly developed LeO-Trap-V1 and those laid in the modified LeO-Trap-V2 by gravid female Ae. aegypti and Ae. albopictus.
Remark: The mean number of eggs in the same row followed by a different letter is significantly different (P < 0.001).
Table 3: Mean number (+ S.E.) of eggs laid by gravid female Ae. aegypti and Ae. albopictus in the modified LeO-Trap-V2 with and without attractant.
Remark: The mean number of eggs in the same row followed by a different letter is significantly different (P < 0.001).
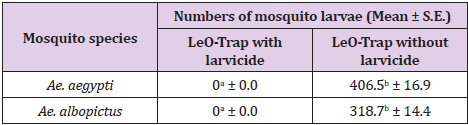
It is interesting to note that no larvae of Ae. aegypti or Ae. albopictus appeared in the LeO-Trap treated with the AZAI larvicide within one week of observation, even though there were many mosquito eggs deposited on the filter papers. In contrast, larvae of Ae. aegypti and Ae. albopictus emerged from the LeO-Trap without larvicide in mean numbers of about 406.5 and 318.7, respectively (Table 4). It is obvious that the AZAI larvicide killed all of the larvae that hatched inside the treated LeO-Trap. The results obtained from these experiments revealed the potential use of the novel lethal ovitrap; the LeO-Trap-V2 with carpet shell extract as an attractant and AZAI® as a larvicide can control new populations of Ae. aegypti and Ae. albopictus mosquitoes.
Table 4: Mean number (+ S.E.) of Ae. aegypti and Ae. albopictus larvae that emerged from the LeO-Trap with and without larvicide.
Remark: The mean number of larvae in the same row followed by a different letter is significantly different (P < 0.001).
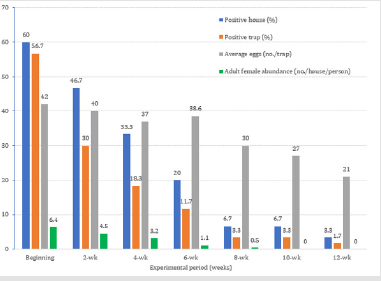
Field evaluations for the oviposition efficacy of the LeO-Trap® against Ae. aegypti mosquitoes were carried out for 12 weeks in 30 houses (2 traps/house) in a village in Muang district, Nonthaburi province, Thailand, and the results are shown in Figure 3. At the beginning of the experiment, 18 houses (60%) were positive for Aedes eggs in 34 LeO-Traps® (56.7%) with a total of 1,428 eggs (average 42 eggs/trap). In the meantime, 230 females Ae. aegypti also were collected from the 18 houses by 2 volunteers using sweep nets (average 6.4 females/house/person). The percentages of positive houses and positive traps decreased dramatically from 46.7% and 30%, respectively, at 2 weeks post start of experiment (2-wk) to about 3.3% and 1.7%, respectively, at the end of the study (12-wk). Similarly, the average numbers of eggs also dropped from 40 eggs/trap (at 2-wk) to 21 eggs/trap (at 12-wk). The hatched larvae from the eggs collected in this experiment were identified as Ae. aegypti. It is interesting to note that no female Ae. aegypti were collected from the houses in the last two assessments (10-wk and 12-wk) of this study.
Figure 3: Field evalution of the LeO-Trap® against Ae. aegypti in 30 houses (2 traps/house) in a village in Muang district, Nonthaburi province, Thailand.
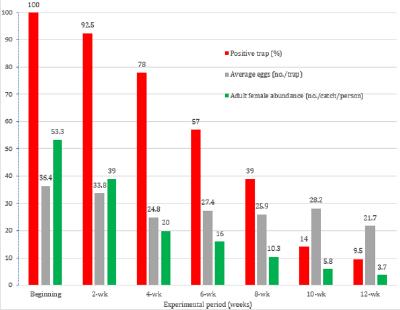
Two hundred LeO-Traps® were evaluated for their oviposition efficacy against Ae. albopictus in a rubber plantation field in Bang Lamung district, Chonburi province, Thailand. It was found that all of the traps (100%) were infested with Aedes eggs at the start of the experiment, with a total of 7,286 eggs (average 36.4 eggs/trap) deposited in the traps (Figure 4). At the same time, a total of 320 female Ae. albopictus also were caught by 6 volunteers within 10 minutes using sweep a net (average 53.3 females/catch/person). The positive traps gradually declined from 92.5% 2 weeks after the start of the study (2-wk) to 78% after 4 weeks (4-wk). Later, the positive traps decreased dramatically to 9.5% at the end of experiment (12-wk). The average numbers of Aedes eggs found in this study fluctuated between 21.7 and 36.4, and they were identified as Ae. albopictus when hatched.
Figure 4: Field evalution of the LeO-Trap® against Ae. albopictus in a rubber plantation in Bang Lamung district, Chonburi province, Thailand.
Discussion
Results obtained from the laboratory experiments demonstrated that the newly designed ovitrap increased oviposition efficacy rates against Ae. aegypti and Ae. albopictus as compared with the conventional ovitrap (Table 1). In addition, the modification of larger windows in the newly designed trap also increased the oviposition efficacy rates against both these Aedes species. This reveals that design of the trap has significant physical influence (Table 2). This trap is totally different from other lethal ovitraps currently available in the markets. In actual fact, the idea of this design came from the experiences of surveys on Aedes larvae in Thai villages. It was noticed that no larvae appeared in outdoor water-storage jars that were without a roof or shade, while the surveys found more Aedes larvae in water-storage jars with partial cover than in those without it, even though they were placed adjacently in the same locations. This could be due to gravid female Aedes trying to seek appropriate containers to lay their eggs with protection from sunlight and rain. It also was found that black seemed to be the most attractive color in the design of the ovitrap. This was confirmed by the results obtained from a study in Florida in 2011, which showed that black ovitraps demonstrated outstanding visual attractiveness over traps of other colors, such as white, blue or orange, or vertical black-and-white stripes [20].
The use of attractant derived from carpet shell extract, in order to lure gravid Ae. albopictus into laying their eggs in the ovitrap, was initiated in Thailand in 2004 by Thavara et. al. [19]. Using the gas chromatography-mass spectrometry (GC_MS) analysis, a total of 83 volatile compounds, such as 3-Methylbutanoic acid, Thiazolidine, Indole, β-Pinene, 1-Octen-3-ol, o-Cresol, etc., were identified from the carpet shell extract (unpublished data). Some compounds among those may act as attractant, but the particularly effective compounds have not been confirmed yet. The carpet shell extract showed excellent oviposition efficacy against Ae. albopictus in laboratory and field evaluations; however, it was limited in practical use, as the extract had to be prepared freshly and used within a short period of 4-5 days. After that it eventually became putrid. These disadvantages became a problem in using the carpet shell extract as attractant, since users rejected its bad smell and inconvenience.
The carpet shell extract in this study was then developed by formulating polymers and adjuvants and preparing it as microcapsule emulsion. This formulation of carpet shell extract is very practical for use without inconvenience for the user, as it is coated inside the trap without an undesirable smell. It also is significant in attracting gravid Ae. aegypti and Ae. albopictus mosquitoes to lay their eggs inside the trap (Table 3). The attractant derived from carpet shell extract used in this study is unique and totally different from previous studies that usually use hay infusion [21-25] or caproic acid [26] as attractant to lure gravid Ae. aegypti and Ae. albopictus. Comparison of oviposition attractiveness against both Aedes species among these attractants would be an interesting issue for further research. The main purpose of the lethal ovitrap is to lure gravid female mosquitoes into laying their eggs inside the trap and eventually ridding the larvae from it. This study incorporated a small amount of about 1 gram of AZAI (1% temephos zeolite granules) into the LeO-Trap® in order to kill the larvae that hatched from the eggs laid inside the trap. In this experiment, we did not count numbers of mosquito eggs attached on the filter papers of both groups of the traps (with and without AZAI) because we did not want to disturb the hatching ability of Ae. aegypti and Ae. albopictus. Therefore, we left the filter papers inside the traps to observe natural hatching of both Aedes species. The data in Table 4 confirmed that the LeO-Trap® with AZAI could kill all of the larvae that hatched inside. Similar result was also obtained from a study conducted in Malaysia in 2015 using temephos as larvicide in the experiment [26]. This study selected AZAI as larvicide to kill the Aedes larvae because it is registered and sold commercially in Thailand at a cheaper price when compared with other larvicides.
In fact, a 100-gram package of AZAI costs about 25 Baht (equal to about 0.82 US$). This cost is economically viable for a commercial ovitrap used for nationwide Aedes mosquito control programs. Temephos is considered to be safe for use in water-storage containers with low toxicity to humans and the environment, as recommended by the World Health Organization (WHO). In addition, the larvicidal efficacy of AZAI could last for at least 3 months after application in water-storage containers in field applications [4-27]. This means that the cost effectiveness of AZAI enables use of the LeO-Trap® for controlling Ae. aegypti and Ae. albopictus offspring in the future. It was the intention of this study to incorporate as little adulticides as possible in the LeO-Trap® in order to minimize the use of insecticides. Many studies revealed the use of adulticides, such as bendiocarb, permethrin, cypermethrin [11], deltamethrin [11,24] and bifenthrin [16] in lethal ovitraps that demonstrated high mortality rates against Ae. aegypti larvae and adults. However, the adulticides used in the lethal ovitrap possibly could harm the users, if contacting it, as well as contaminate the environment and adversely affect non-target organisms. In addition, adulticides could diminish oviposition efficacy of the lethal ovitrap. For example, bifenthrin caused fewer Ae. aegypti eggs in the lethal ovitraps when compared to lethal ovitraps without insecticides [16].
A prototype of lethal ovitrap derived from injection larvicide (pyriproxyfen) into molding of ovitrap made from low density polyethylene was produced in 2016 and its lethal effect was carried out against Ae. aegypti [28]. Surprisingly, this innovative lethal ovitrap showed complete emergence inhibition against the mosquitoes for a long period of at least 30 weeks. It could be an excellent lethal ovitrap for controlling Ae. aegypti when it is available in the market. Recently in 2017, a study in Florida showed the potential use of larvicide (pyriproxyfen) and biological adulticide (Beauveria bassiana spores) in the lethal ovitrap to control Ae. aegypti and Ae. albopictus under semi-field conditions [13]. This could be an effective lethal ovitrap to control both Aedes species when the similar results are confirmed in field evaluations. In our field studies, many predators, such as spiders, lizards and small toads were observed in many LeO-Trap® when we opened them to collect the filter papers for counting of mosquito eggs. These predators stayed in the traps to feed mosquitoes or other insects that flew into the traps. This means they are biological control animals for adult mosquitoes in the LeO-Trap® without application of any adulticides. It also confirms that LeO-Trap® has no adverse effects on non-target organisms in the surrounding areas of application. In contrast, a study in Australia demonstrated that the sticky ovitrap applied as lethal ovitrap to control Ae. aegypti could impact on non-target insects as only 2.2% out of the total captured specimens were Ae. aegypti [29]. Therefore, the environmental impacts, especially on non-target organisms must be considered carefully when any applications of vector control are carried out.
The LeO-Trap® demonstrated excellent efficacy against Ae. aegypti and Ae. albopictus in the field, as can be seen from the data in Figures 3&4. It could collect many eggs of both species in the study areas, in which large numbers of mosquito offspring were reduced in following generations. This was confirmed by the female abundance of both species, which declined consecutively over the experimental period of 3 months. Obviously, the LeO-Trap® is an effective device that can reduce the populations of Ae. aegypti and Ae. albopictus mosquitoes in the future. Therefore, it should be used as early as April or May before rainy season begins in Thailand. However, the LeO-Trap® alone cannot control Aedes mosquito populations completely, and it should be used as an additional tool to mosquito fish, larvicide application in water-storage containers and adulticide application (if necessary) in the integrated vector control program for controlling DHF. A study in Thailand conducted during 1999 and 2000 confirmed that adult Ae. aegypti populations were reduced partially by using a lethal ovitrap, but its efficacy was lower than expected, due to numerous waters holding containers being competitive oviposition sites [14]. Elimination of alternative oviposition containers in surrounding areas would increase efficacy of the lethal ovitraps [14-16].
The field experiment conducted in urban houses in Nonthaburi used only two traps in each house, which collected numerous Ae. aegypti eggs and reduced the abundance of females dramatically by the end of the experiment. However, this would not be a sufficient number of ovitraps for controlling Ae. aegypti mosquitoes and stopping transmission of DHF in endemic areas. Studies in north Queensland, Australia, in 2006 [15] and southern Puerto Rico in 2014 [17] suggested that about 3-4 ovitraps should be deployed in each house for controlling Ae. aegypti populations satisfactorily. It is obvious that success of ovitrap deployment comprises high coverage of targeted houses at least 80%, elimination of alternative breeding containers, participation of people in the communities, and the use of effective lethal ovitraps [18].
The lethal ovitrap has been called the LeO-Trap® since 2016, and was registered for Patent at the Department of Intellectual Property, Ministry of Commerce, with proprietary right between the Department of Medical Sciences, Ministry of Public Health, Thailand and Ikari Trading (Thailand) Co., Ltd. This was the outcome of cooperative research between government agencies and the private sector, which was carried out according to the “Pracharath” policy of the Thai Government, in order to encourage the private sector to create an innovative product that could benefit public health and the Thai economy under academic and technological support of the government agencies. The LeO-Trap® has been sold commercially to Thai users by the Ikari Trading (Thailand) Co., Ltd. via various channels and social media. The instructions and explanations for home use of the LeO-Trap® are demonstrated in Figure 5. The success of this research in reducing Aedes mosquito offspring has made people eager to purchase the LeO-Trap® for their homes. On a few occasions, some customers complained that the LeO-Trap® did not work because many live mosquitoes still flew around their houses; however, it was found that these mosquitoes were Culex quinquefasciatus Say, as a result of our intensive investigations. Many customers had expected the LeO-Trap® to kill all kinds of mosquitoes, but this was a misunderstanding because the LeOTrap ® was designed to control only Aedes mosquitoes according to their biology and oviposition preference. The Aedes larvae hatched from eggs laid in the LeO-Trap® would be dead because of AZAI, and some dead larvae and adult Ae. aegypti might be observed (Figure 6).
Figure 5: Instructions and explanations for users of the LeO-Trap® for controlling Aedes mosquitoes at home.
Figure 6: Dead female Ae. aegypti and her eggs found in the LeO-Trap® (photos courtesy of the Vector Borne Diseases Division, Department of Disease Control).
In practice, the carpet shell attractant in microcapsule emulsion could provide attractive efficacy against Ae. aegypti and Ae. albopictus for about 3 months in the field; however, it is apparent that LeO-Trap® could collect Aedes eggs even though it has been used for over a year. In other words, LeO-Trap® could be used to lure gravid Ae. aegypti and Ae. albopictus as a long time because of its physical attractiveness. The most important things for using LeO-Trap® are refilling of water every 2-3 weeks and replacement of AZAI every 3 months. All lethal ovitraps become useless when they are dry or without insecticides.
Conclusion
In conclusion, the results obtained from this study revealed that the LeO-Trap® showed excellent efficacy in luring gravid female Ae. aegypti and Ae. albopictus into laying their eggs in the trap, and all of the larvae that hatched from the eggs were killed eventually by AZAI: the zeolite granules containing temephos at 1%. The LeO-Trap® is an innovative device that is economically and environmentally friendly for controlling Ae. aegypti and Ae. albopictus populations. Therefore, it can be used as an additional tool in the integrated vector control program for controlling Aedesborne diseases in Thailand and elsewhere. However, further field studies on the impact of the LeO-Trap® on reducing the incidence of DHF, Zika and chikungunya infection in applicable areas should be carried out.
Acknowledgement
This study was supported by the Department of Medical Sciences, Ministry of Public Health, Thailand. We appreciated kind help of Chitti Chansang, National Institute of Health, Department of Medical Sciences, to provide mosquito larvae for laboratory testing.
References
- Bannister BA, Begg NT, Gillepie SH (1996) Infectious Disease. London: Blackwell Science.
- (2012) WHO. Global Strategy for Dengue Prevention and Control 2012-2020: World Health Organization.
- Lardeux FJ (1992) Biological control of culicidae with the copepod mesocyclops aspericornis and larvivorous fish (poeciliidae) in a village of french polynesia. Med Vet Entomol 6(1): 9-15.
- Thavara U, Tawatsin A, Kong Ngamsuk W, Mulla MS (2004) Efficacy and longevity of a new formulation of temephos larvicide tested in village-scale trials against Aedes aegypti larvae in water-storage containers. J Am Mosq Control Assoc 20(2): 176-182.
- Thavara U, Tawatsin A, Asavadachanukorn P, Mulla MS (2009) Field evaluation in Thailand of spinosad, a larvicide derived from Saccharopolyspora spinosa (Actinomycetales) against Aedes aegypti (L.) larvae. Southeast Asian J Trop Med Public Health 40(2): 235-242.
- Thavara U, Tawatsin A, Chansang C, Asavadachanukorn P, Zaim M, et al. (2007) Simulated field evaluation of the efficacy of two formulations of diflubenzuron, a chitin synthesis inhibitor against larvae of Aedes aegypti (L.) (Diptera: Culicidae) in water storage containers. Southeast Asian J Trop Med Public Health 38(2): 269-275.
- Marini L, Baseggio A, Drago A, Martini S, Manella P, et al. (2015) Efficaccy of two common methods of application of residual insecticide for controlling the Asian tiger mosquito, Aedes albopictus (Skuse), in urban areas. PloS ONE 10(8): e0134831.
- Zeichner BC, Debboun M (2011) The lethal ovitrap: A response to the resurgence of dengue and chikungunya. US Army Med Dep J p. 4-11.
- Achee NL, Gould F, Perkins TA, Reiner RC Jr, Morrison AC, et al. (2015) A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis 9(5): e0003655.
- Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, et al. (2013) Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit Vectors 6: 280.
- Zeichner BC, Perich MJ (1999) Laboratory testing of a lethal ovitrap for Aedes aegypti. Med Vet Entomol 13(3): 234-238.
- Parker CN, Pereira RM, Baldwin RW, Chaskopoulou A, Koehler PG (2017) Laboratory evaluation of a novel lethal ovitrap for control of Aedes aegypti. J Med Entomol 54(6): 1666-1673.
- Buckner EA, Williams KF, Marsicano AL, Latham MD, Lesser CR (2017) Evaluating the vector control potential of the IN2CARE mosquito trap against Aedes aegypti and Aedes albopictus under semifield conditions in Manatee County, Florida. J Am Mosq Control Assoc 33(3): 193-199.
- Sithiprasasna R, Mahapibul P, Noigamol C, Perich MJ, Zeichner BC, et al. (2003) Field evaluation of a lethal ovitrap for the control of Aedes aegypti (Diptera: Culicidae) in Thailand. J Med Entomol 40(4): 455-462.
- Williams CR, Long SA, Russell RC, Ritchie SA (2006) Optimizing ovitrap use for Aedes aegypti in Cairns, Queensland, Australia: Effects of some abiotic factors on field efficacy. J Am Mosq Control Assoc 22(4): 635-640.
- Williams CR, Ritchie SA, Long SA, Dennison N, Russell RC (2007) Impact of a bifenthrin-treated lethal ovitrap on Aedes aegypti oviposition and mortality in north Queensland, Australia. J Med Entomol 44(2): 256-262.
- Barrera R, Amador M, Acevedo V, Caban B, Felix G, et al. (2014) Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J Med Entomol 51(1): 145-154.
- Johnson BJ, Ritchie SA, Fonseca DM (2017) The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects 8(1): E5.
- Thavara U, Tawatsin A, Chompoosri J (2004) Evaluation of attractants and egg-laying substrate preference for oviposition by Aedes albopictus (Diptera: Culicidae). J Vector Ecol 29(1): 66-72.
- Hoel DF, Obenauer PJ, Clark M, Smith R, Hughes TH, et al. (2011) Efficacy of ovitrap colors and patterns for attracting Aedes albopictus at suburban field sites in north-central Florida. J Am Mosq Control Assoc 27(3): 245-251.
- Allan SA, Kline DL (1995) Evaluation of organic infusions and synthetic compounds mediating oviposition in Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J Chem Ecol 21(11): 1847-1860.
- Obenauer PJ, Allan SA, Kaufman PE (2010) Aedes albopictus (Diptera: Culicidae) oviposition response to organic infusions from common flora of suburban Florida. J Vector Ecol 35(2): 301-306.
- Gopalakrishnan R, Das M, Baruah I, Veer V, Dutta P (2012) Studies on the ovitraps baited with hay and leaf infusions for the surveillance of dengue vector, Aedes albopictus, in northeastern India. Trop BioMed 29(4): 598-604.
- Quimbayo M, Rúa Uribe G, Parra Henao G, Torres C (2014) Evaluation of lethal ovitraps as a strategy for Aedes aegypti control. Biomedica 34(3): 473-482.
- Velo E, Kadriaj P, Mersini K, Shukullari A, Manxhari B, et al. (2016) Enhancement of Aedes albopictus collections by ovitrap and sticky adult trap. Parasit Vectors 9: 223.
- Ong SQ, Jaal Z (2015) Investigation of mosquito oviposition pheromone as lethal lure for the control of Aedes aegypti (L.) (Diptera: Culicidae). Parasit Vectors 8: 28.
- Tawatsin A, Thavara U, Chompoosri J, Bhakdeenuan P, Asavadachanukorn P (2007) Larvicidal efficacy of new formulations of temephos in non-woven sachets against larvae of Aedes aegypti (L.) (Diptera: Culicidae) in water-storage containers. Southeast Asian J trop Med Public Health 38(4): 641-645.
- Harburguer L, Licastro S, Masuh H, Zerba E (2016) Biological and chemical characterization of a new larvicide ovitrap made of plastic with pyriproxyfen incorporated for Aedes aegypti (Diptera: Culicidae) Control. J Med Entomol 53(3): 647-652.
- Long SA, Jacups SP, Ritchie SA (2015) Lethal ovitrap deployment for Aedes aegypti control: Potential implications for non-target organisms. J Vector Ecol 40(1): 139-145.

 Research Article
Research Article