Abstract
Background: Inflammatory Myofibroblastic Tumor (IMT) is a very rare neoplasm. When Endoscopic Gastroduodenoscopy (EGD) was performed, submucosal tumors of stomach were found incidentally. According to different sources of layer and echogenicity in the Endoscopic Ultrasonography (EUS) finding, submucosal tumors like carcinoid, pancreatic rest, Gastrointestinal Stromal Tumor (GIST), leiomyoma and schwannoma wound be distinguished. However, IMTs are one of submucosal tumors in the stomach. We must put this impression in the differential diagnosis when performing EUS.
Case Summary: We present a 55-year-old woman without symptoms who received a health examination, and had a gastric tumor was found during EGD. Initial biopsies showed chronic inflammation. Positron Emission Tomography (PET) showed an increased Fludeoxy Glucose (FDG) uptake in the stomach. Endoscopic ultrasonography was also performed. After surgical intervention, pathological analysis identified an inflammatory myofibroblastic tumor. No recurrence was observed by EGD or a PET scan during the follow-up. The relevant literature from the PubMed database was reviewed, and the clinical presentation, laboratory data, treatment strategies and outcomes of 42 reported cases were analyzed. Forty-two patients with gastric IMTs showed a female predominance (female: male: 26: 16). The most common location of gastric IMTs were gastric bodies (18 of 42). The most common symptoms were abdominal pain (21 of 42). Only two cases were asymptomatic. Tumor recurrence was found in 3 cases after surgical intervention in the reviewed literature.
Conclusion: EUS is useful to identify submucosal tumor in the gastrointestinal tract. IMTs must keep in mind when performing EUS.
Keywords: Non-Diabetes; Coronary Heart Disease (CHD); Lipoprotein(a) [Lp (a)]; Hemoglobin A1c(HbA1c); Gensini Score
Abbreviations: CHD: Coronary Heart Disease; ACS: Acute Coronary Syndrome; SCD: Stable Coronary Heart Disease; HbA1c: Hemoglobin A1c; Lp(a): Lipoprotein a; Fbg: Fibrinogen; Hcy: Homocysteine; CRP: C-Reactive Protein; AS: Atherosclerosis; TG: Triglyceride; FBG: Fasting Blood Glucose
Introduction
Inflammatory Myofibroblastic Tumor (IMT) is a very rare neoplasm usually seen in young people and children [1]. While the lung is the most common site [2]. IMT is rarely observed in the stomach especially in adults. Gastric IMTs, which shows a female predominance, may present with a variety of symptoms depending on the location of the tumor. The difficulty in radiologically differentiating between malignant and benign lesions imposes another clinical challenge to clinicians. In this report, we present a case of a gastric IMT in an asymptomatic woman.
Case Presentation
Brief history
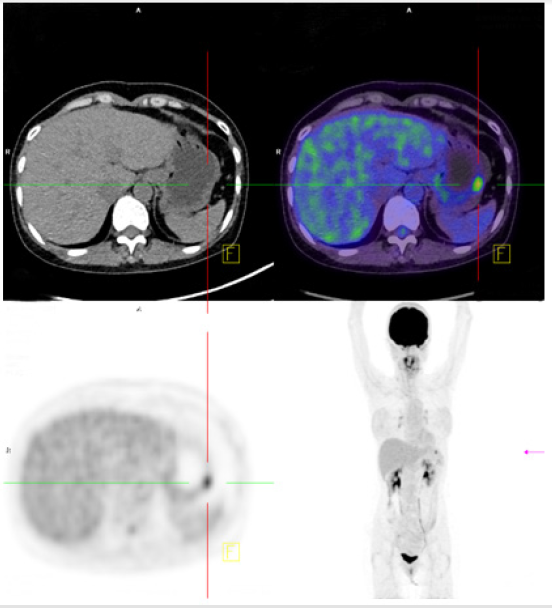
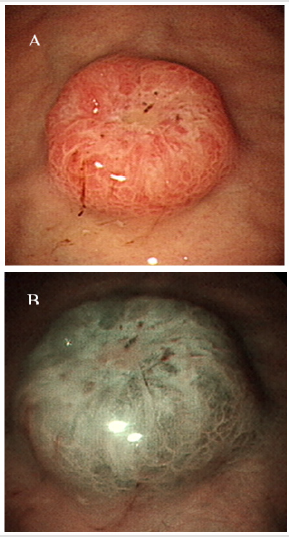
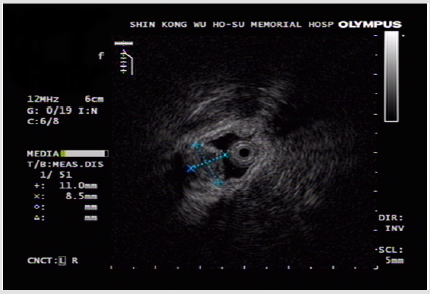
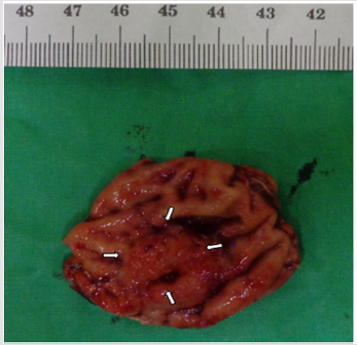
A 55-year-old woman without known systemic disease underwent a physical check-up in May 2014. Basic laboratory studies gave normal values (Hb: 12.3gm/dL, WBC: 7100/μL, INR: 1.09). Besides, liver and renal functions as well as levels of tumor markers including CEA, CA199, alpha-fetoprotein were within normal limits. There were no abdominal discomfort, no nausea or vomiting, no weight loss or other discomfort recently. No hypertension or diabetes mellitus were found in her family history. She was allergy to pyrine. No surgical history was found. On the other hand, PET scan showed increased Fludeoxy Glucose (FDG) uptake over the greater curvature of the stomach (Figure 1). Endoscopic gastroduodenoscopy revealed a reddish tumor with central depression about 1cm over the greater curvature of midbody (Figures 2a & 2b). Endoscopic ultrasound study demonstrated focal thickening of muscular layer with loss of layering over the first, second and third layers (Figure 3) for which biopsy was taken. Pathologic analysis showed chronic inflammation with infiltration of lympho-plasma cells. The initial impression was Gastrointestinal Stromal Tumor (GIST) or leiomyoma. After discussion with the pathologist, IMT was diagnosed. We discussed with patient about treatment for this tumor. The patient agreed to receive therapeutic endoscopy for tumor resection. After a futile attempt of local endoscopic resection due to technical problem, surgical intervention of subtotal gastrectomy with gastroduodenostomy was performed (Figure 4). After stabilization of general condition, the patient was discharged with regular follow-ups at the outpatient clinic. There was no evidence of tumor recurrence after following the patient for 24 months by endoscopic gastroduodenoscopy and PET scan. No adverse and unanticipated events were found during follow-up.
Figure 1: 1.21cm nodule (max SUV: 6.21, delayed SUV: 12.05) with increased FDG uptake was found above the greater curvature of the stomach. SUV: standard uptake value.
Figure 2: Tumor features by endoscopic gastroduodenoscopy. A: A 1 cm mass was found above the greater curvature of the mid-body (white light view). B: It is suspected to be a submucosal tumor in the Narrow Band Imaging (NBI) view.
Figure 3: Submucosal tumor, up to 1cm in size, with focal thickening of the muscular layer and loss of layering over the 1st, 2nd and 3rd layers observed by endoscopic ultrasound (EUS).
Pathology
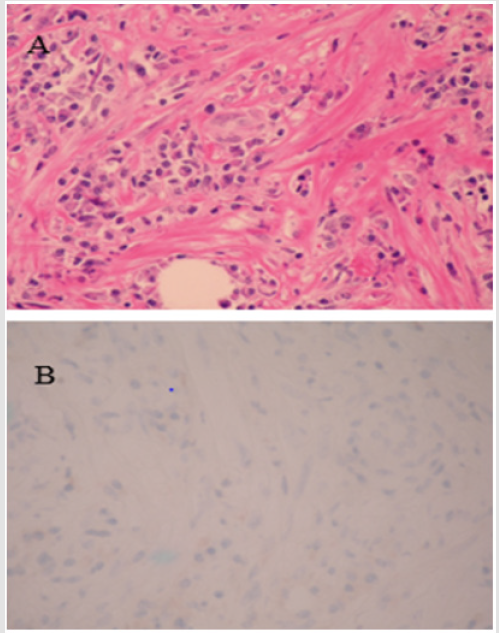
Microscopically, the tumor was mainly located at the submucosa with an infiltrating border. The overlying mucosa was ulcerated. The tumor was composed of bland-looking myofibroblasts and lympho-plasma cells within a fibrotic and collagenized stroma (Figure 5a). No abnormal mitotic figure or tumor necrosis was found. Immunohistochemical staining showed CD68-positive but ALK-negative myofibroblasts (Figure 5b). The diagnosis of an ALKnegative inflammatory myofibroblast tumor was made.
Figure 5: A: Features of the IMT by microscopy. A: The tumor is composed of bland looking myofibroblasts and lymphoplasma cells within a fibrotic and collagenized stroma. B: ALK staining was negative.
Final Diagnosis
The final diagnosis was IMT which was origin from Muscularis propria layer.
Outcome and Follow-up
After stabilization to general conditions, the patient was discharged with regular follow-ups at the outpatient clinic. There was no evidence of tumor recurrence after following the patient for 24 months by endoscopic gastroduodenoscopy and PET scan. No adverse and unanticipated events were found during follow-up.
Discussion
Background Review
Inflammatory Myofibroblastic Tumor (IMT), also known as inflammatory pseudotumor, is rare [3]. IMT is usually seen in children and young adults with a slight male predominance (male: female, 1.4: 1) [4,5]. Gastric IMT, which has been found to show a female predominance (male: female = 1: 4) [6,7], is even rarer. Although the lung is most commonly affected, IMT has been reported to involve other organs such as the liver, pancreas, spleen, lymph node, breast, kidney, bladder, orbits, and central nervous system [8]. It has been hypothesized that IMT is associated with uncontrolled inflammation from Epstein-Barr virus, human herpesvirus-8, E. coli, H. pylori, or cytomegalovirus infections as well as gastroesophageal reflux disease [9]. Moreover, recent studies imply that some IMTs are related to IgG4 [10]. Although IMT may be asymptomatic, symptoms related to its location might be observed [5]. IMT has been reported to be associated with fever, malaise, weight loss, anemia, and thrombocytosis but can also be asymptomatic [5], depending on the location of the tumor in the stomach [11]. Gastric IMT may also spread to adjacent organs and cause various symptoms, which usually subside after tumor resection.
Literature Review
All of the English literature in the PubMed database from January 1989 to December 2017 was searched using the key words “Positron emission tomography”, “Gastric inflammatory myofibroblastic tumor”, and “Inflammatory pseudotumor”. The demographic (i.e., age and gender) and clinical manifestations, laboratory findings, locations and sizes of tumor, pathological features of biopsied specimens, treatment strategies and follow-ups of the reported patients were reviewed. Non-English literature and reports on patients younger than 18 years of age were excluded. All statistical analyses were performed using commercially available SPSS software (version 15.0 for Windows; SPSS, Chicago, IL, Unites States). Data are expressed as the mean ± SD. The results are summarized in Table 1.
Patient Demography and Clinical Characteristics of Gastric Imts
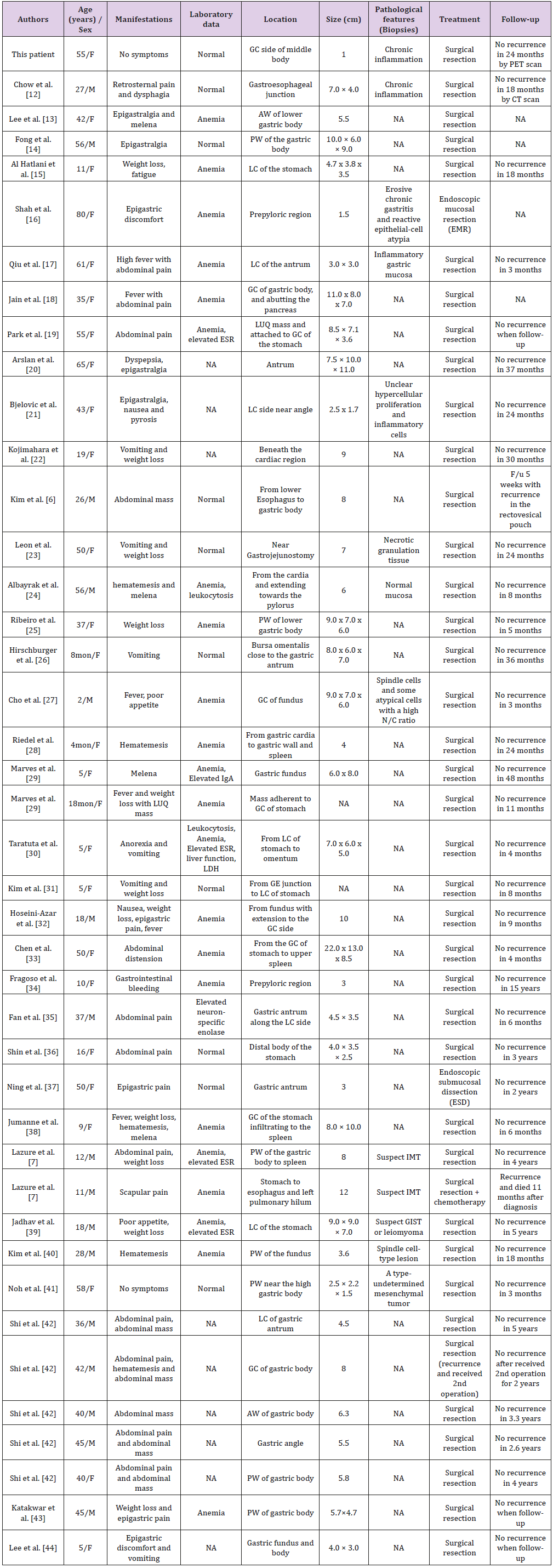
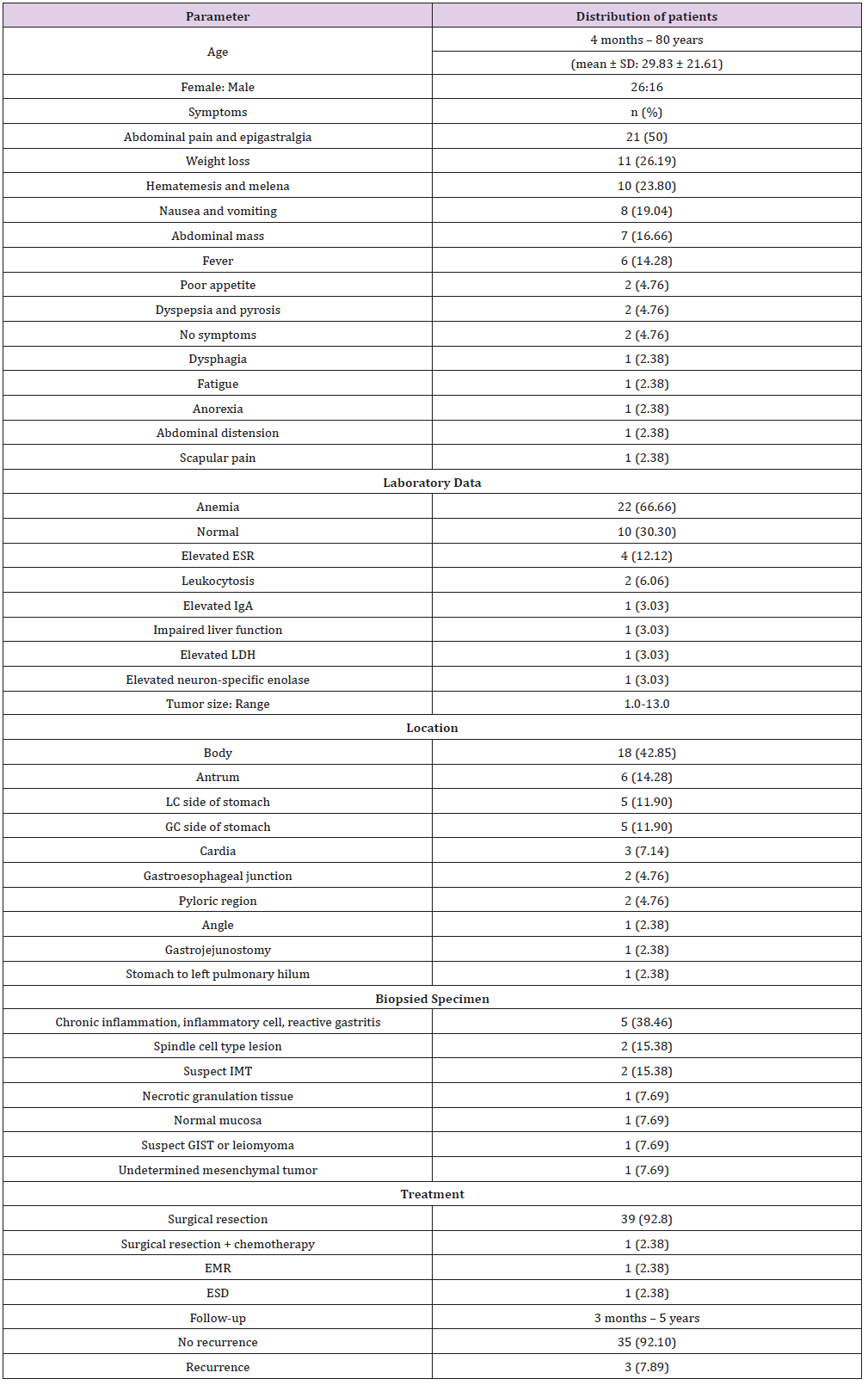
A review of the patient demography, clinical manifestations, tumor characteristics, and treatments of 42 previously reported cases of gastric IMTs (Table 1) showed a female predominance (female: male, 26: 16) and a mean age of 29.83±21.61 years (ranging from 4 months – 80 years old). The most common symptoms were abdominal pain, followed by vomiting (or nausea), body weight loss, hematemesis (or melena), fever, dysphagia, dyspepsia (or pyrosis), palpable abdominal mass, poor appetite or anorexia, and fatigue. The symptoms are related to the locations of the tumors. Laboratory studies revealed that anemia was the most common anomaly in these patients (22 out of 33). Other abnormalities included elevated Erythrocyte Sedimentation Rate (ESR), leukocytosis, elevated IgA, impaired liver function, elevated LDH, and elevated neuron-specific enolase. The most common location of tumors during endoscopic examinations was the gastric body (42.9%) (18 of 42). Other reported locations of gastric IMTs included the antrum, cardia, gastroesophageal junction, angle, and site of gastrojejunostomy. Chronic inflammation or inflammatory cells (5 of 13) was the most common finding upon pathological examination of biopsied specimens among the reviewed literature in which a biopsy was performed. (Table 2), possibly due to the subepithelial location of the tumor.
Table 1: Summary of the demographics and clinical characteristics of reported inflammatory myofibroblastic tumor (IMT) cases.
The most common treatment for gastric IMT is surgery (40 of 42). The other two patients (2 of 42) were treated by endoscopic submucosal dissection (ESD) and endoscopic mucosal resection (EMR). One surgical patient (1 of 40) was treated by surgical resection plus chemotherapy. The range of follow-up was 3 months to 5 years. Tumor recurrence was found in three patients (3 of 42) with gastric IMT upon follow-up. One of these three patients was treated with surgical resection plus chemotherapy and died after tumor recurrence during follow-up. Tumor recurrence, including carcinomatosis, was found in another patient. The third patient had a 2nd surgical resection upon tumor recurrence. There was no recurrence after the 2nd operation during follow-up. Additionally, the findings of the female predominance, most common tumor site, and major treatment are compatible with previous studies [1,6,7- 39]. The summarized clinical features are shown in Table 2.
Radiological Diagnosis
There is no radiological sign that serves as a diagnostic basis for gastric IMT [40-45]. Gastric IMTs have been reported to present as heterogeneous, lobar, calcified, cystic lesions on ultrasound and non-enhanced abdominal CT examinations [1], though they may manifest as homogeneous or heterogeneous lesions with central delayed enhancement and peripheral early filling [45,46]. Abdominal CT may help to determine the invasion of gastric IMTs to adjacent organs [6].
Endoscopic Diagnosis
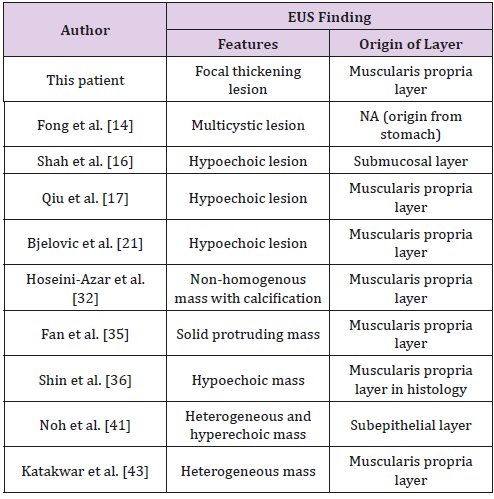
The diagnostic role of Endoscopic Ultrasound (EUS) remains unclear in previous clinical settings. From the reviewed literature, EUS was only performed in a few patients. We identified patients who underwent EUS, and they are summarized in Table 3. Ten patients in the reviewed literature underwent EUS before surgical treatment. The most common feature in the EUS results was a hypoechoic mass (5 of 10). Other features such as a hyperechoic, heterogenous lesion were also reported in the reviewed literature. The most common IMT origin layer is the muscularis propria layer (7 of 10). The submucosal and subepithelial layers were also reported in the literature. The differential diagnosis of submucosal tumors arising from the muscularis propria layer included Gastrointestinal Stromal Tumor (GIST), leiomyoma, and schwannoma. Hypoechoic lesions observed through echogenicity were found in these submucosal tumors. According to the most common EUS results for IMT in the reviewed literature, IMT with a hypoechoic lesion arising from the muscularis propria layer might cause a differential diagnosis. To our best knowledge, this is the first article to discuss the features of IMT by EUS.
PET Diagnosis
In our patient, the diagnosis was based on positron emission tomography. Dong et al. has previously reported a mean SUVmax of 10.9±5.5 for IMT, ranging from 3.3 to 20.8 [47]. The wide variability, which is believed to be attributed to varying proportions of inflammatory cells in the tumor [47], renders PET suboptimal for differentiating IMTs from other tumors. By contrast, PET appears to be a useful tool for the follow-up of IMT relapse after treatment, which is typically reflected by an elevation of SUVmax. In our case, the SUVmax of the gastric IMT was 6.21. The four factors that have been found to affect the tissue uptake of fluorodeoxyglucose by PET include tumor cellularity, the biological behaviors of tumor cells, the composition and proportion of inflammatory cells, and the degree of inflammatory cell activation [47].
Histological Features
The typical histological features of IMT include myofibroblastic proliferation with lymphoplasmacytic infiltration in myxoid background stroma [48]. IMT is known to have the potential for malignant transformation and metastasis. Recurrence, which is not uncommon for incompletely resected lesions, has been reported to occur in 7% (3 in 38) of patients with a mortality rate of 5% (2 in 38) [49]. Local recurrence of IMT has been shown to be related to the rearrangement of the anaplastic lymphoma kinase (ALK) gene on chromosome 2p23 [4]. Indeed, chromosomal translocations of active ALK gene have been found in almost 50% of IMTs. Previous studies have indicated that ALK gene translocation, which may be associated with a higher recurrence rate, mostly occurs in children and young adults. By contrast, IMTs without an ALK gene translocation are mostly found in older people and are associated with distal metastasis. Moreover, cellular atypia, a ganglion-like cell morphology, aneuploidy, and overexpression of p53 have been reported to be associated with tumor aggressiveness [4,50]. Consistently, our patient showed no evidence of an ALK gene translocation. The positivity of staining for cytokeratin, laminin, calponin, smooth muscle actin (SMA), muscle-specific actin (MSA), fibronectin, and desmin also varies in IMTs and cannot provide reliable diagnostic clues [12].
Treatment Strategies and Prognosis
Complete surgical resection is the most efficient treatment for gastric IMTs. Among the gastric IMT patients, two were treated with endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD), while the rest underwent surgical resection. The indication of EMR or ESD for a particular patient was due to the relatively small size of the tumor together with its favorable location for the endoscopic approach. Incompletely resected IMTs have been reported to have a high one-year local recurrence rate [6,51] with an overall probability of recurrence up to 60% [5,52]. Survival after complete resection is 91% at 5 years and 77% at 10 years [51]. Partial resection may be considered when complete resection is inappropriate for selected patients with severe co-morbidities to whom radiotherapy or chemotherapy (cyclosporine, methotrexate, azathioprine, and cyclophosphamide) may be applied for the relief of symptoms [4,8]. Recent advancements in IMT treatment include the use of an anaplastic lymphoma kinase (ALK) inhibitor, which has been reported in two patients with abdominal and pancreatic IMTs with recurrence after surgical resection, one of whom showed a partial response. The other patient was withdrawn from the trial due to disease progression [53]. The benefit of including an ALK inhibitor as a standard therapeutic agent against IMTs remains to be elucidated.
Conclusion
Albeit uncommon, the diagnosis of gastric IMT should not be ruled out upon encountering asymptomatic gastric tumors during endoscopic examination. Gastric IMT might give rise to a differential diagnosis when hypoechoic lesions arise from the muscularis propria layer by EUS. Although the major treatment for gastric IMT is surgical resection, due to the high recurrence rate for incompletely resected tumors, chemotherapy and radiotherapy may be considered for patients with unresectable lesions for symptom relief. Translocation of the ALK gene may play an important role and may be associated with outcomes. An ALK gene inhibitor might provide new treatment options for IMTs with recurrence in the future.
References
- Karnak I, Senocak ME, Ciftci AO, Caglar M, Bingol Kologlu M, et al. (2001) Inflammatory myofibroblastic tumor in children: diagnosis and treatment. J Pediatr Surg 36(6): 908-912.
- Hedlund GL, Navoy JF, Galliani CA, Johnson WH (1999) Aggressive manifestations of inflammatory pulmonary pseudotumor in children. Pediatr Radiol 29(2): 112-116.
- Papachristou GI, Wu T, Marsh W, Plevy SE (2004) Inflammatory pseudotumor of the liver associated with Crohn's disease. J Clin Gastroenterol 38(9): 818-822.
- Coffin CM, Hornick JL, Fletcher CD (2007) Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 31(4): 509-520.
- Coffin CM, Watterson J, Priest JR, Dehner LP (1995) Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol 19(8): 859-872.
- Kim KA, Park CM, Lee JH, Cha SH, Park SW, et al. (2004) Inflammatory myofibroblastic tumor of the stomach with peritoneal dissemination in a young adult: imaging findings. Abdom Imaging 29(1): 9-11.
- Lazure T, Ferlicot S, Gauthier F, Doz F, Couturier J, et al. (2002) Gastric inflammatory myofibroblastic tumors in children: an unpredictable course. J Pediatr Gastroenterol Nutr 34(3): 319-322.
- Narla LD, Newman B, Spottswood SS, Narla S, Kolli R (2003) Inflammatory Pseudotumor. RadioGraphics 23(6): 719-729.
- Privette A, Fisk P, Leavitt B, Cooper K, Mc Cahill L (2008) Inflammatory myofibroblastic tumor presenting with esophageal obstruction and an inflammatory syndrome. Ann Thorac Surg 86(4): 1364-1367.
- Stone JH, Zen Y, Deshpande V (2012) IgG4-related disease. N Engl J Med 366(6): 539-551.
- Behranwala KA, Straker P, Wan A, Fisher C, Thompson JN (2005) Inflammatory myofibroblastic tumour of the gallbladder. World J Surg Oncol 3(1): 24.
- Chow SC, Nahal A, Mayrand S, Ferri LE (2010) Pulmonary inflammatory myofibroblastic tumor invading the gastroesophageal junction. Ann Thorac Surg 89(5): 1659-1661.
- Lee YK, Wang HY, Shyung LR, Chang CW, Chen MJ (2011) Inflammatory myofibroblastic tumor: an unusual submucosal lesion of the stomach. Endoscopy 43 Suppl 2 UCTN: 151-152.
- Fong AKW, Teoh AYB, Chiu PWY, Wong SKH, Ng EKW (2010) Gastric inflammatory myofibroblastic tumor masquerading as a pancreatic cystic neoplasm. Endoscopy 42: E231-E232.
- Al Hatlani M, Ratcliffe EM (2010) Endoscopic visualization of a gastric polypoid mass: a rare pediatric presentation of an inflammatory myofibroblastic tumor. Gastrointest Endosc 72(4): 894-895.
- Shah SM, Sussman D, Jorda M, Ribeiro A (2008) EUS with EMR of an inflammatory myofibroblastic tumor of the stomach. Gastrointest Endosc 67(3): 561-563.
- Qiu J, Shi Y, Fang L, Wang H, Zhang M (2014) Case report-high fever as an initial symptom of primary gastric inflammatory myofibroblastic tumor in an adult woman. Int J Clin Exp Med 7(5): 1468-1473.
- Jain A, Kasana S, Ramrakhiani D, Sharma M (2012) Inflammatory myofibroblastic tumor of the stomach in an adult female report of a rare case and review of the literature. Turk J Gastroenterol 23(4): 399-405.
- Park SH, Kim JH, Min BW, Song TJ, Son GS, et al. (2008) Exophytic inflammatory myofibroblastic tumor of the stomach in an adult woman: a rare cause of hemoperitoneum. World J Gastroenterol 14(1): 136-139.
- Arslan D, Gunduz S, Tural D, Uysal M, Tatli AM, et al. (2013) Inflammatory myofibroblastic tumor: a rarely seen submucosal lesion of the stomach. Case Rep Oncol Med pp. 328108.
- Bjelovic M, Micev M, Spica B, Babic T, Gunjic D, et al. (2013) Primary inflammatory myofibroblastic tumor of the stomach in an adult woman: a case report and review of the literature. World J Surg Oncol 11: 35.
- Kojimahara K, Mukai M, Yamazaki K, Yamada T, Katayama T, et al. (1993) Inflammatory pseudotumor of the stomach: report of a highly infiltrative case with electron microscopic and immunohistochemical studies. Acta Pathol Jpn 43(1-2): 65-70.
- Leon CJ, Castillo J, Mebold J, Cortez L, Felmer R (2006) Inflammatory myofibroblastic tumor of the stomach: an unusual complication after gastrectomy. Gastrointest Endosc 63(2): 347-349.
- Albayrak F, Dursun H, Albayrak Y, Altas S, Uyanik A, et al. (2010) Inflammatory myofibroblastic tumor of the stomach in an adult woman: a rare intermittent cause of gastric outlet obstruction. Tumori 96(3): 492-495.
- Ribeiro MCB, Lopes LR, Neto JCDS, Meirelles LR, de Carvalho RB, et al. (2012) Rare Gastric inflammatory myofibroblastic tumor in an adult woman: a case report with review of the literature. Case Rep Med 374070.
- Hirschburger M, Enders J, Alzen G, Padberg W, Wagner HJ (2010) An inflammatory myofibroblastic tumor of the stomach as a rare cause of gastric outlet obstruction in an 8-month-old infant. Klin Padiatr 222(3): 192-193.
- Cho MY, Min YK, Kim NR, Cho SJ, Kim HK, et al. (2002) Fever of unknown origin as a presentation of gastric inflammatory myofibroblastic tumor in a two-year-old boy. J Korean Med Sci 17(5): 699-703.
- Riedel BD, Wong RC, Ey EH (1994) Gastric inflammatory myofibroblastic tumor (inflammatory pseudotumor) in infancy: case report and review of the literature. J Pediatr Gastroenterol Nutr 19(4): 437-443.
- Maves CK, Johnson JF, Bove K, Malott RL (1989) Gastric inflammatory pseudotumor in children. Radiology 173(2): 381-383.
- Taratuta E, Krinsky G, Genega E, Roche K, Geneisier N (1996) Pediatric inflammatory pseudotumor of the stomach: contrast-enhanced CT and MR imaging findings. AJR Am J Roentgenol 167(4): 919-920.
- Kim HB, Maller E, Redd D, Hebra A, Davidoff A, et al. (1996) Orthotopic liver transplantation for inflammatory myofibroblastic tumor of the liver hilum. J Pediatr Surg 31(6): 840-842.
- Hoseini Azar MM, Mokhtare M, Zare Mirzaie A, Gholami A, Agah S, et al. (2016) Fever,weight loss and early satiety due to gastric inflammatory myofibroblastic tumor; case report and literature review. Middle East J Dig Dis 8(2): 138-142.
- Chen WC, Jiang ZY, Zhou F, Wu ZR, Jiang GX, et al. (2015) A large inflammatory myofibroblastic tumor involving both stomach and spleen: a case report and review of the literature. Oncol Lett 9(2): 811-815.
- Fragoso AC, Eloy C, Estevao Costa J, Campos M, Farinha N, et al. (2011) Abdominal inflammatory myofibroblastic tumor a clinicopathologic study with reappraisal of biologic behavior. J Pediatr Surg 46(11): 2076-2082.
- Fan J, Huang B, Yang X, Yang M, He J, et al. (2017) ALK-positive gastric inflammatory myofibroblastic tumor in an adult with familial adenomatous polyposis and diffuse fundic polyposis. Diagn Pathol 12(1): 68.
- Shin HC, Gu MJ, Kim SW, Kim JW, Choi JH (2015) Coexistence of gastrointestinal stromal tumor and inflammatory myofibroblastic tumor of the stomach presenting as a collision tumor: first case report and literature review. Diagn Pathol 10: 181.
- Ning X, Liang B, Liu Q, Chen X, Xu M (2017) Gastric inflammatory myofibroblastic tumor identified as ectopic pancreas and treated by endoscopic submucosal dissection. Gastrointest Endosc 86(5): 921-922.
- Jumanne S, Shoo A, Mumburi L, Akoko L, Scanlan P, et al. (2017) Gastric inflammatory myofibroblastic tumor presenting as fever of unknown origin in a 9-year-old girl. Eur J Gastroenterol Hepatol 29(1): 68-72.
- Jadhav M, Harvi R, Patil R, Kittur S (2019) Inflammatory myofibroblastic tumor of the stomach presenting as an exophytic mass - a diagnostic dilemma. Turk Patoloji Derg 35(2): 151-156.
- Kim DJ, Kim W (2015) Inflammatory myofibroblastic tumor treated with laparoscopic proximal gastrectomy and double-tract anastomosis. J Gastric Cancer 15(1): 64-67.
- Noh BJ, Min JW, Sung JY, Park YK, Lee J, et al. (2016) Inflammatory myofibroblastic tumor-like stromal proliferation within gastric inverted hyperplastic polyp. Pathol Int 66(3): 180-182.
- Shi H, Wei L, Sun L, Guo A (2010) Primary gastric inflammatory myofibroblastic tumor: a clinicopathologic and immunohistochemical study of 5 cases. Pathol Res Pract 206(5): 287-291.
- Katakwar A, Gedam BS, Mukewar S, Agasti A (2014) Primary gastric inflammatory myofibroblastic tumor in an adult-case report with brief review. Indian J Surg Oncol 5(1): 66-70.
- Lee D, Suh YL, Lee SK (2006) Calcifying fibrous pseudotumour arising in a gastric inflammatory myofibroblastic tumour. Pathology 38(6): 588-591.
- Uysal S, Tuncbilek I, Unlubay D, Tiras U, Bilaloglu P, et al. (2005) Inflammatory pseudotumor of the sigmoid colon mesentery: US and CT findings (2004:12b). Eur Radiol 15(3): 633-635.
- Slavotinek JP, Bourne AJ, Sage MR, Freeman JK (2000) Inflammatory pseudotumour of the pancreas in a child. Pediatr Radiol 30(11): 801-803.
- Dong A, Wang Y, Dong H, Gong J, Cheng C, et al. (2014) Inflammatory myofibroblastic tumor: FDG PET/CT findings with pathologic correlation. Clin Nucl Med 39(2): 113-121.
- Coffin CM, Dehner LP, Meis Kindblom JM (1998) Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: an historical review with differential diagnostic considerations. Semin Diagn Pathol 15(2): 102-110.
- Makhlouf HR, Sobin LH (2002) Inflammatory myofibroblastic tumors (inflammatory pseudotumors) of the gastrointestinal tract: how closely are they related to inflammatory fibroid polyps? Hum Pathol 33(3): 307-315.
- Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM (1999) Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol 12(3): 279-286.
- Takeda S, Onishi Y, Kawamura T, Maeda H (2008) Clinical spectrum of pulmonary inflammatory myofibroblastic tumor. Interact Cardiovasc Thorac Surg 7(4): 629-633.
- Cerfolio RJ, Allen MS, Nascimento AG, Deschamps C, Trastek VF, et al. (1999) Inflammatory pseudotumors of the lung. Ann Thorac Surg 67(4): 933-936.
- Butrynski JE, D'Adamo DR, Hornick JL, Dal Cin P, Antonescu CR, et al. (2010) Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 363(18): 1727-1733.

 Case Report
Case Report