Abstract
Hydroxyapatite (HAP-- Ca10(PO4)6(OH)2) is a biocompatible and bioactive material that is widely used for biomedical applications, especially in bone replacements. It has good load carrying capacity; however, it lacks antibacterial property. New bio-composites based on bovine hydroxyapatite doped with, magnetite iron oxide (HAP/ Fe3O4) matrix reinforced with ZnO and MgO nanoparticles are proposed for biomedical applications that provide improved antibacterial activity with potential to be used in magnetic therapy. Microwave sintering was used to manufacture the composites. The microstructure evolution in these composites were studied by Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS). Density, microhardness, compressive strength of the composites was measured and compared along with their magnetic properties. Finite element analysis simulations were performed for the compression tests.
Keywords: Natural Hydroxyapatite; MagneticFe3O4; Biocompatibility; SEM & EDS Analyses; Bio Composites; ZnO & MgO Additions
Introduction
Recently, many advances in the synthesis and surface engineering of iron oxide nanoparticles have been made [1]. Moreover, there are still newly formed magnetic nanoparticles proposed (bio glass ceramics [2,3-10] and hydroxyapatite [4]) [5]. Hydroxyapatite (HAP), being the main mineral phase of natural bone, is a commonly studied material for biomedical applications [6-8]. It is commonly used in bone grafting and tissue engineering applications due to its excellent biocompatibility and osteon-conductivity [9]. HAP, Ca10(PO4)6(OH)2, has a hexagonal crystal lattice structure [10-28], which allows for a wide variety of substitutions by anions, cations, and functional groups, such as the F- [11], Fe2+/3+ [4, 12-16], and CO2− [17]. Iron is of interest as a cation that can be substituted in HAP due to the fact it is naturally present in trace amounts in both teeth and bone [13]. Furthermore, its presence provides iron substituted apatite (FeHA) with possible magnetic properties that can potentially be applied to various applications, including drug delivery, medical imaging, or hyperthermia based cancer therapies, for which pure HAP is unsuitable [1,14,18-22]. Magnetic therapy has been considered as a promising treatment alternative in health care, especially in the treatment of bone diseases. Research has indicated that magnetic fields may stimulate the proliferation and differentiation of osteoblasts, promote the expression of growth factors such as bone morphogenetic protein, increase osteonintegration, and accelerate new bone formation [23-26]. Magnetic fields were also found to be beneficial in promoting the integration of bone and implants, increasing bone density and calcium content, and accelerating the healing of bone fractures [27-30].
Among the magnetic materials usually used in the biomedical field, Magnetic Nanoparticles (MNPs) have drawn great interest owing to their unique magnetic properties, including the fact that they become superparamagnetic at diameters of < 20 nm [31]. Although the role of iron in bone accrual has received little attention, a few studies have previously shown that iron restriction can have an inhibitory effect on the mineralization of osteoblasts in vitro and experimental evidence also suggests that there may be some positive association between iron metabolism and the in vitro proliferation of bone or non-bone cell lines [32-37]. Additionally, the implant associated infection is widely considered as a major concern in the field of biomedical applications and this has been the driving force for developing HAP-based biomaterials with antibacterial additives for possible use in prosthetic devices.
In our present work, we sintered Hydroxyapatite (HAP) with different concentrations of zinc oxide micro rods (ZnO) at 1250°C to produce HAP-ZnO bio composites. In vitro antimicrobial studies were carried out to understand how ZnO addition (up to 30 wt %) to HAP leads to the improvement in bacteria static/bactericidal property and thereby reduce bacterial infection on implant surface. Some of the biomedical based research have shown that addition of ZnO also had a modest influence on fracture toughness and hardness as well as improving the antimicrobial property. A maximum up to 1.7 MPam1/2 indentation fracture toughness and hardness of up to 6.8 GPa were measured in HAP- ZnO biocomposites [38-51]. Other researches have also shown that the additions of reinforcing elements like ZnO and MgO in HAP/magnetic iron oxide composites could reduce the bacterial infections on the surface of the composites and increase their hardness which is a positive feature for different medical implants [2,10,11].
For this reason, the current work aims to present the design of new bio-composites based on bovine hydroxyapatite (BHA)/ nano-magnetite iron oxide (Fe3O4) reinforced with ZnO and MgO nanoparticles. Here, in the frame of the “Bio ceramic” research project, a net shape microwave sintering procedure was used by using a few percent of paraffin to create a natural micro porous structure as an alternative to the other materials to create a porous structure. A special attention was given for the microstructural evolution with recently developed compositions to give practical significance for the application as biomaterials. Mechanical and other physical-chemical characteristics were studied in detail. The structure evolutions of these composites were observed in details by means of Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS).
Experimental Conditions
HAP-Materials Processing and Microwave - Sintering
Natural HAP was obtained from calcinated fresh - young bovine bones (femurs) by following the method developed at SUPMECA/ LISMMA-Paris [2,10]. The femurs were undergone deproteinization with NaOH treatment. After repeated washing, they were heat treated at 850°C. The treated HAP powder (particle sizes of 1-2μm) was the mixed with other constituents as described in Sec 2.2. In this work, microwave sintering process has been carried out for the manufacturing of the bio-composite materials details of which were given in earlier papers [2,10,11]. The application of microwave energy to the processing of various materials such as ceramics, metals and composites offers several advantages over conventional heating methods. Microwave heating results in lower energy costs and decreased processing times for many industrial processes. These advantages
Include unique microstructure and properties, improved product yield, energy savings, reduction in manufacturing cost and synthesis of new materials. In order to compare the microstructural evolution of microwave sintered composites to the ones manufactured by conventional sintering, some specimens were sintered in an electrical - conventional furnace (High Temperature Furnace. In this project, the primary aim, however, is to use a house type microwave oven under laboratory conditions. For this reason, a house type (2.45 GHz) microwave oven was modified to be used in the manufacturing process. A special thermocouple, insulated for the microwaves, was installed to monitor the temperature during production. The accuracy of temperature measurement with this device was determined to be within -10°C of the temperature measured. The thermocouples were placed inside the alumina ceramic crucible, 2-3 mm away from the specimens. Compared to the conventional sintering, there was a slight increase in density of the specimens manufactured with microwave sintering. Since the microwave sintering took much shorter time, densification rate in the microwave sintering process may be higher than in the conventional sintering. Effective sintering time for these samples was chosen as 30 minutes.
Specimen Design and other Experimental Design
The compact geometry was prepared based on the matrix natural HAP + 20wt% magnetic iron oxide (Fe3O4) reinforced with different percentages of nano MgO and ZnO (received from VWR-France). At the beginning of the process, a pre-treatment of doping of iron oxide was made with the reinforcements for surface activation and for increasing of homogeneous distribution of the reinforcements in the matrix. For this treatment, a very simple process has been carried out: pre-mixing and pre-heating of the magnetic iron oxide (Fe3O4) with the reinforcement at 100-150°C followed by surface activation with hydrogen peroxide during the mixture at this temperature.
Then, the blended powders were homogenized by ball milling for two hours, then compacted by uniaxial cold isostatic pressing at a pressure of 300 MPa, intending to produce an initial green density ranging 85-90%. Cylindrical test specimens were prepared (Height=11mm, Diameter=11mm) according to the British Standards-BS 7253. In the second stage, micro hardness and quasi static compression tests were performed on the sintered samples to study the influence of microstructure and phase composition on the micro-mechanical behaviour of the manufactured composites. For microstructural surface analysis acetic and lactic acids were used to etch the surfaces. Microstructure was evaluated by SEM and chemical analysis by “EDS” analysis. Fracture surfaces of the specimens after the compression tests were also evaluated by SEM. Magnetic measurements were carried out by the physicalchemistry research laboratory in Paris. Two test specimens were used for each composite determine the magnetic saturation values. The results for the different composites were compared to each other. Experimental results were used to create a simple Finite Element (FEM) analysis model to study the behaviour of the bio ceramic materials during deformation.
Results and Discussions
Microstructural Evaluation
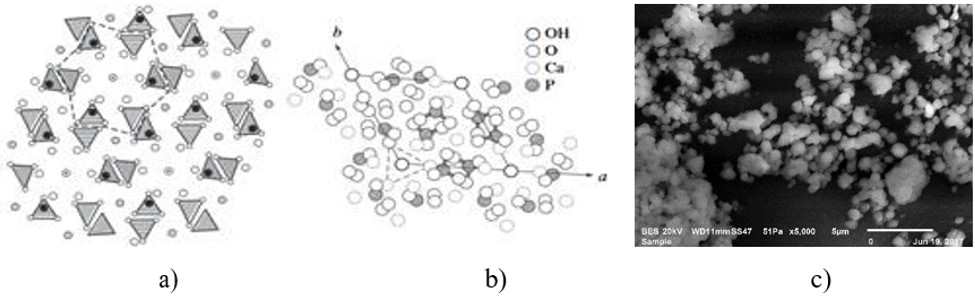
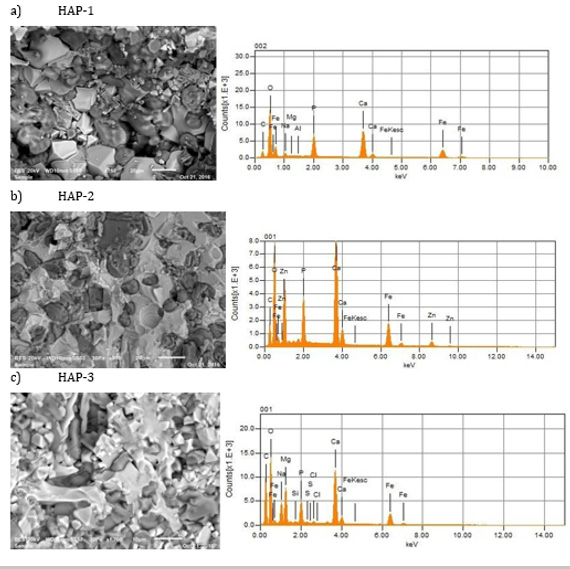
General compositions of the HAP based Biocomposites are given in the Table 1. In the frame of the common research project, only three composites were presented here. Basically, HAP was doped with pure magnetic Fe3O4 after those secondary reinforcements were added as explained in the former section. HAP has a hexagonal structure with lattice parameters a = 0.942 nm and c = 0.687 nm. The ideal formula of HAP is given as Ca10(PO4)6(OH)2. The atomic structure of HAP and its projection along the “c” axis are shown in Figure 1. Also, Figure 1c shows the average grain size measured of this mixture which varies from 1 to 5μm. This structure verifies that the microwave sintering is a viable manufacturing method with lower energy costs and shorter processing times for these materials. Figure 2 shows the microstructures of natural HAP + 20% magnetic iron oxide (Fe3O4) reinforced with different percentages of nano ZnO (10%) and MgO (10%) taken from the SEM image with “EDS” analysis that was distributed homogeneously in the structure for three compositions prepared here.
Figure 1: a) Atomic structure of HAP, b) its projection along the c axis [38, 39] and HAP powders after 2 hours of milling used in this work
Figure 2: Microstructures of three composites obtained after sintering (1200°C, 2 hours), for HAP-1, HAP-2 and HAP-3, respectively.
Mechanical - Physical Behaviour; Density, Microhardness and Compression Tests
A main idea in the improvement of the mechanical and physical properties of the implant materials is their mechanical strength. Bio ceramics should have strength like bone tissue that also exhibit a good fatigue and fracture toughness under both static and dynamic forces. In the literature, the modulus of elasticity of the bone varies from 0.005 to 0.5 GPa, depending on its position and its age [38- 42]. Simultaneously, the elastic modulus of certain ceramics for medical applications is around 380 GPa. Again, it is known that the resultant stress gradient may be the origin of the fracture along the bone implant interface [38]. Finally, it is not good idea to use very hard implant materials that can increase the rate of bone wear. In the frame of this present work, all the density measurements of the specimens were carried out by using Archimedes method. These values varied between 1.79 ± 0.15 g/cm3 for HAP-1 and 2.05 ± 0.25 g/cm3 for HAP-2 and 2.19 ± 0.30 g/cm3 for HAP-3 respectively. Again, microhardness tests results measured on the 3 specimens for each composite were found as 95 ± 0,25 HVN for HAP-1 and 225 ± 0,12 HVN for HAP-2 and finally 321 ± 0,18 HVN for HAP-3 respectively. Quasi-static compression test was performed on 4-5 specimens for each composition with a servo-hydraulic MTS Universal test system (model: 5500R) at an initial rate of 10 mm/min and second rate of 5 mm/min.
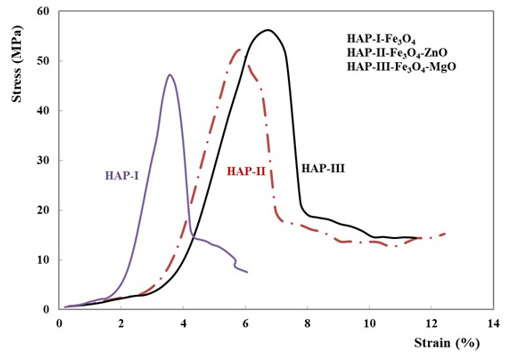
Maximum load endpoint was 5000N. Quasi-static compression test results have been shown in the Figure 3. As shown in the Figure 3, the third series (HAP-3) have shown a little bit higher strength but is more ductile then the two others (HAP-1 and HAP- 2). Fracture surfaces of these three composites are shown in Figure 4. All of these pictures justify that microwave sintering give a good solution. A good cohesion in each structure is observed on the broken specimens. Influence of MgO on the fracture behaviour is considerable which was also given in the literature [2,10,38,39,43].
Figure 3: Static compression test results for three composites developed in this work, HAP-1, HAP-2 and HAP-3 respectively.
Figure 4: Fracture surfaces after the compression test for three composites, HAP-1, HAP-2 and HAP-3 respectively.
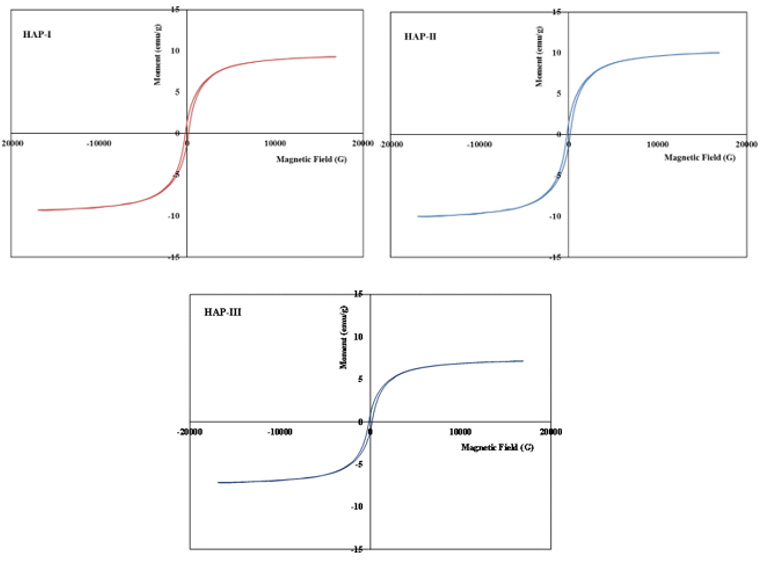
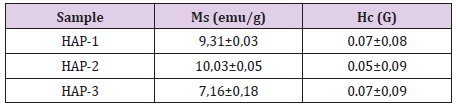
Evaluation of Magnetic Properties for HAP-1, HAP-2 and HAP-3
Magnetic measurements have been carried out by the physicalchemistry research laboratory in Paris. Two test specimens were used for each composite and evaluated for finding the magnetic saturation values (Figure 5) and compared certain parameters and summarized in the Table 2. As well known, magnetic properties of the composite structures can be improved with small grain size distribution well in the matrix especially in the nanoscale [2,10,17,21,23,42-55]. As indicated in the former papers [2,10,11,17,21,23,38,39,42-55], a basic and important parameter in the characterization of the magnetic materials is the power loss; this kind of power gives a measure of the energy density available in the material for a specific application. Magnetic measurements of the Fe3O4/HAP based composites have shown very similar quasi super- paramagnetic behaviour; they give magnetic saturation between 7.87 and 8.66 emu/g.
Figure 5: Fracture surfaces after the compression test for three composites, HAP-1, HAP-2 and HAP-3 respectively.
As known well that the magnetic coercivity (coercivity force) measures the ability of a ferromagnetic material to withstand an external magnetic field without becoming demagnetized. This value measured for three composites here is variable between 0, 05 to 0.07 kA/m (Oe). For this reason, these values measured here is very efficient for biomedical applications of these composites. It seems that all of the three composites very similar value of Ms to be maximized as much as possible to confirm a better response under the application of the pieces. These results should be considered as the indicative values for three composites and other measurements for these composites are going on in the frame of the research project.
Finite Element Simulation of the Microstructure of HAP Based Composites
The idealized microstructure considered in this work consists of a random arrangement of cylindrical inclusions embedded in a continuous HAP bio composite matrix (HAP+Fe3O4). The volume fraction of the reinforcements is varied from 5%, 10% to 20% and the micro-macro transition schemes are evaluated in many case. However, when the volume fraction increases, nearby the reinforcements start to interact and this can influence the overall mechanical behaviour.
The distributions of the reinforcements in the matrix were considered randomly and as equal shape and they were aligned in similar way. The Representative Volume Element (RVE) microstructure is periodic along the 3 directions, permitting us to put on periodic boundary conditions to the external faces of the specimens. The positioning of the reinforcements is controlled by the practical limitation of producing a suitable FE mesh. A simple condition is useful to the minimal distance between each inclusion surface and the external faces of the specimen. The volume of one particular cell for the reinforcements is less than 1 mm3.
In the same way, the representative cells are also meshed with quadratic tetrahedral. FE simulations are achieved using ABAQUS (2008-Supmeca-Paris) and the whole volume is meshed using 4-node C3D4 tetrahedral in ABAQUS), allowing us to improved detention the strain gradients in the matrix. After that, the prediction was efficiently compared to those obtained with finer meshes. It means that comparison of active response is made to the regular response of the reinforcement. Figure 6 indicates typical meshes formation generated for a composite with various percentages of the reinforcements and variable geometry of specimens that were used in this work.
Evidently, the macroscopic stress predicted by the FE analysis is
computed from an average volume of the stress tensor given at each
integration point over the RVE of domain “i”. The same method was
applied as have been done in the former papers of the authors [2,10]:
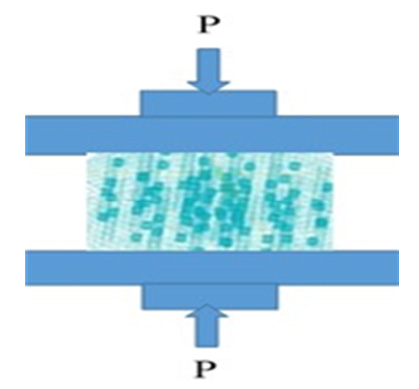
Figure 6: Schematic of experimental setup and position of the composite in FEM (HAP+Fe3O4 additionally reinforced with MgO and ZnO).
As for the procedure of numerical part, it is intended to calculate the macroscopic stresses and strains using a homogenization procedure of the numerical part. This procedure simply consists on defining the deformation state at each integration point, in the FE model, as well as the present matrix average state that depends on the corresponding state at the previous step time, etc. As known, a simple FE analysis proposed here affords an alternative approach for approximating of the material properties. A simple FE model is proposed here for the heterogeneous trial in which the material parameters are considered as variable. The simulation is then accomplished, and results can be compared to data obtained from comparable experimental results. The agreement between the model predictions and the data is quantified and judged to be adequate or not. If the settlement is not satisfactory, the parameter values can be updated after that a new FE model can be produced and run. At the final stage, the process carries on until obtaining satisfactory results. This simple method constructed here can give understandable and comparable predictions regarding to the experimental results.
Additionally, boundary conditions considered here for the material properties should be arranged: it means that a simple boundary condition is Ux, Uy, Uz = 0 at the bottom border and also, a negative displacement load Uy in y-direction was imposed at the upper face of the specimen to make through the rigid plate indenter (see Figure 5 for experimental setup and the position of the HAP and reinforcement in the FEM for these composites). A static step with small step time is used to assess the gradual evolution of stresses and strains in the basics model; here, the corresponding reaction force is calculated and used to supply the maximum load.
Certain mechanical properties of the composites designed in the present work are then predicted from the reinforcement properties. Hydroxyapatite (HAP) bio composite ceramics reinforced basically with magnetic iron oxide (Fe3O4), and secondly added reinforcements are pure pulverised MgO and ZnO powders respectively.
The reinforcement materials is assumed to be linearly elastic with elastic modulus. Ep =359 GPa with a density of ρ = 5.12 g/cm3 and poison’s ratio υ=0.12 for fine magnetic iron oxide (Fe3O4) as a main part of the matrix. Again, Ep =330 GPa with a density of ρ = 3.56 g/cm3 and poison’s ratio υ=0.35 for pure MgO and Ep =120 GPa with a density of ρ = 5.6 g/cm3 and poison’s ratio υ=0.34 for pure ZnO. As a basic data considered for the model designed in the present work. The FE solution used to simulate multiple-phase composites consisting of an elastic-plastic matrix reinforced by linear elastic inclusions. Uniaxial compressive loading is successively applied to the multi particle cells embedded in the HAP matrix. The average of the macroscopic strain over a RVE computed at each time step provides the loading history for the corresponding FE models.
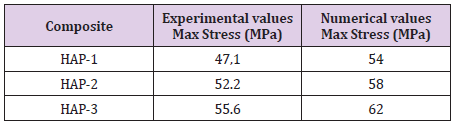
Average equivalent stress in the inclusions of multiple-phase composite materials is determined for different volume fractions of the reinforcing phase. With multiple reinforcements, FE predictions (FE with 25-30 % volume fraction) correspond to a uniaxial compression test. The predictions of the compressive results were compared with experimental compression test results by using of deformation and maximum stress values (Table 3). The average volume fractions for the reinforcements were indicated in the same table. For the sake of simplicity, only compressive test results were considered by using a rigid plate.
Table 3: Comparison of maximum experimental and numerical stress values (MPa) for three different compositions.
As for these comparisons, numerical values were found higher than those of experimental. Naturally, experimental conditions in laboratory scale are influenced with many artificial reasons occurred during the test. Position, porosity, geometry of the specimen and other environmental conditions can influence these results whereas numerical results obtained by a simple FE model here give the results considered for ideal perfect specimens. It should be more precious experimental work. These results give a practical solution and give idea for industrial applications of these biomaterials. For this reason, these results should be accepted as indicative results as a helpful tool for manufacturing of new design of biocomposites.
Conclusion
A basic idea in this work is centralized in the improvement of the mechanical and physical properties of the bio composites used as implant materials. For this reason, new designs of HAP based bio composites were developed by using a microwave sintering. As is well known, the addition of reinforcing elements such as ZnO and MgO in HAP/magnetic iron oxide composites could reduce the bacterial infections on the surface of the composites and increase their hardness and strength which is a positive feature for different medical implants. In the present work, the results obtained with the ZnO and MgO reinforcements should be considered as indicative values and can help for the manufacturing of these types of bio composites. As for FEM solution, the reaction of the reinforcements (considered as inclusions here) in the HAP matrix can be predicted based on the solution of the reinforcement in a finite medium having the properties of the matrix. Here only a simple FEM allowing heterogeneous field was proposed to solve an equivalent inclusion problem.
Macroscopic deformation histories corresponding to the non-monotonic uniaxial and the plane strain compression were consecutively considered. Here, a simple prediction has made by using a simple FEM Naturally, development of HAP bio composites needs more investigations to attempt a high accuracy between experimental results and their equivalent FE predictions.
Acknowledgment
The authors acknowledge for the financial support for this research given by Supmeca/Paris-FR and UNICAMP/FEMCampinas/ SP-BR.
References
- Gupta AK, M Gupta (2005) Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26(18): 3995-4021.
- D Katundi, F Ayari, E Bayraktar, A Tosun Bayraktar (2011) Experimental and Finite Element Analysis (FEM) of Bio ceramics, Experimental and Applied Mechanics 6: 13-20.
- Min Hua Chen, Chung King Hsu, Feng Huei Lin, Leszek Stobinski, J Peszke (2006) Folic Acid Immobilized Ferrimagnetic DP-Bioglass to Target Tumor Cell for Cancer Hyperthermia Treatment. Advances in Science and Technology 53: 50-57.
- His Chin W, W Tzu Wei, S Jui Sheng, W Wen Hsi, L Feng Huei (2007) A novel biomagnetic nanoparticle based on hydroxyapatite. Nanotechnology 18(16): 165601.
- Hou CH, SM Hou, YS Hsueh, J Lin, HC Wu, et al. (2009) The in vivo performance of biomagnetic hydroxyapatite nanoparticles in cancer hyperthermia therapy. Biomaterials 30(23-24): 3956-3960.
- Martini FH (2006) Fundamentals of Anatomy & Physiology. (7th Edition) San Francisco: Benjamin Cummings.
- Weiner S, HD Wagner (1998) The Material Bone: Structure-Mechanical Function Relations. Annual Review of Materials Science 28(1): 271-298.
- Park JB, JD Bronzino (2003) Biomaterials: Principles and Applications CRC Press: Boca Raton pp. 250.
- Elliott JC (1994) Preface, in Studies in Inorganic Chemistry, JC. Elliott, Editor, Elsevier. v-vii.
- D Katundi, F Ayari, E Bayraktar (2011) Design of Natural Hydroxyapatite as bio‐composite ceramics (HAP): Experimental and Numerical Study, AIP Conference Proceedings 1315, 235.
- Pawlak Z, Pai R, Bayraktar E, Kaldonski T, Oloyede A (2008) Lamellar lubrication in vivo and vitro: friction testing of hexagonal boron nitride. Bio Systems 94: 202-208.
- Jiang M, J Terra, AM Rossi, MA Morales, EMB Saitovitch, DE Ellis (2002) Fe2+/Fe3+ substitution in hydroxyapatite: Theory and experiment. Phys Rev B p. 66.
- Kay MI, RA Young, AS Posner (1964) Crystal Structure of Hydroxyapatite. Nature 204 (4963): 1050-1052.
- Morrissey R, LM Rodríguez Lorenzo, KA Gross (2005) Influence of ferrous iron incorporation on the structure of hydroxyapatite. Journal of Materials Science: Materials in Medicine 16(5): 387-392.
- Gross KA, R. Jackson JD Cashion, LM Rodriguez Lorenzo (2002) Iron substituted apatites: A resorbable biomaterial with potential magnetic properties in 4th International Conference on the Scientific and Clinical Applications of Magnetic Carriers, M Alini, et al., Editors. 2002, Swiss Society for Biomaterials: Switzerland. 114 - 117.
- Prakash KH, R Kumar, C Ooi, T Sritharan, Cheang, KA Khor (2006) Wet-chemical synthesis and magnetic property studies of Fe(III) ion substituted hydroxyapatite. Molecular and Cellular Biomechanics 3(4): 177-178.
- Cazalbou S, D Eichert, X Ranz, C Drouet, C Combes, MF Harmand, C Rey (2005) Ion exchanges in apatites for biomedical application. Journal of Materials Science: Materials in Medicine 16(5): 405-409.
- Pankhurst QA, NTK Thanh, SK Jones, J Dobson (2009) Progress in applications of magnetic nanoparticles in biomedicine. Journal of Physics D: Applied Physics 42(22): 224001.
- Duguet E, S Mornet, S Vasseur, JM Devoisselle (2006) Magnetic nanoparticles and their applications in medicine. Nanomedicine 1(2): 157-168.
- Jain TK, J Richey, M Strand, DL Leslie Pelecky, CA Flask, et al. (2008) Magnetic nanoparticles with dual functional properties: Drug delivery and magnetic resonance imaging. Biomaterials 29(29): 4012-4021.
- Lu AH, EL Salabas,F Schüth (2007) Magnetic Nanoparticles: Synthesis, Protection, Functionalization, and Application. Angewandte Chemie International Edition 46(8): 1222- 1244.
- Kramer E, M. Zilm, M Wei (2013) A Comparative Study of the Sintering Behavior of Pure and Iron- Substituted Hydroxyapatite. Bioceramics Development and Applications 3(1).
- Bassett CA, M Schink Ascani, SM Lewis (1989) Effects of pulsed electromagnetic fields on Steinberg ratings of femoral head osteonecrosis. Clin Orthop Relat Res (246): 172-85.
- Santini MT, G Rainaldi, A Ferrante, PL Indovina, Vecchia, et al. (2003) Effects of a 50 Hz sinusoidal magnetic field on cell adhesion molecule expression in two human osteosarcoma cell lines (MG-63 and Saos-2). Bioelectromagnetics 24(5): 327-338.
- McLeod KJ, L Collazo (2000) Suppression of a Differentiation Response in MC-3T3-E1 Osteoblast- Like Cells by Sustained, Low-Level, 30 Hz Magnetic-Field Exposure. Radiation Research 153(5): 706-714.
- Jansen JH, O van der Jagt, BJ Punt, JA Verhaar, J van Leeuwen, et al. (2010) Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: an in vitro study. BMC Musculoskelet Disord 11: 188.
- Fini M, R Cadossi, V Canè, F Cavani, G Giavaresi, et al. (2002) The effect of pulsed electromagnetic fields on the osteointegration of hydroxyapatite implants in cancellous bone: a morphologic and microstructural in vivo study. Journal of Orthopaedic Research 20(4): 756-763.
- Zhang Xy, Y Xue, Y Zhang (2006) Effects of 0.4 T rotating magnetic field exposure on density, strength, calcium and metabolism of rat thigh bones. Bioelectromagnetics 27(1): 1-9.
- Chang K, WHS Chang (2003) Pulsed electromagnetic fields prevent osteoporosis in an ovariectomized female rat model: A prostaglandin E2-associated process. Bioelectromagnetics 24(3): 189-198.
- Taylor KF, N Inoue, B Rafiee, JE Tis, KA McHale, et al. (2006) Effect of pulsed electromagnetic fields on maturation of regenerate bone in a rabbit limb lengthening model. Journal of Orthopaedic Research 24(1): 2-10.
- Zeng XB, H Hu, LQ Xie, F Lan, W Jiang, et al. (2012) Magnetic responsive hydroxyapatite composite scaffolds construction for bone defect reparation. International Journal of Nanomedicine 7: 3365-3378.
- Le NTV, DR Richardson (2004) Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood 104(9): 2967-2975.
- Parelman M, B Stoecker, A Baker, D Medeiros (2006) Iron Restriction Negatively Affects Bone in Female Rats and Mineralization of hFOB Osteoblast Cells. Experimental Biology and Medicine 231(4): 378-386.
- Pawlak Z, Kaldonski T, Pai R, Bayraktar E, Oloyede A (2009) A comparative study on the tribological behaviour of hexagonal boron nitride (h-BN) as lubricating micro particles - an additive in porous sliding bearings for a car clutch. Wear 267: 1198-1202.
- Panseri S, C Cunha, T D’Alessandro, M Sandri, G Giavaresi, et al. (2012) Intrinsically superparamagnetic Fe-hydroxyapatite nanoparticles positively influence osteoblast-like cell behaviour. Journal of Nanobiotechnology 10(1): 32.
- Takegami K, T Sano, H Wakabayashi, J Sonoda, T Yamazaki, et al. (1998) New ferromagnetic bone cement for local hyperthermia. Journal of Biomedical Materials Research 43(2): 210-214.
- Wang J, T Nonami, K Yubata (2008) Syntheses, structures and photophysical properties of iron containing hydroxyapatite prepared by a modified pseudo-body solution. Journal of Materials Science: Materials in Medicine 19(7): 2663-2667.
- V Orlovskii, VS Komlev, SM Barinov (2002) Hydroxyapatite and Hydroxyapatite-Based Ceramics, Inorganic Materials 38(10): 973-984.
- W Wei, W Song, S Zhang (2014) Preparation and characterization of hydroxyapatite-poly (vinyl alcohol) composites reinforced with cellulose nanocrystals, Bio Resources 9(4): 6087-6099.
- D Lahiria, V Singhb, A Benaducec, S Sealb, L Kosc, A Agarwal (2011) Boron nitride nanotube reinforced hydroxyapatite composite: Mechanical and tribological performance and in-vitro biocompatibility to osteoblasts. J mechanical behaviour of biomedical materials 4(1): 44-56.
- Qu H, AL Vasiliev, M Aindow, M Wei (2005) Incorporation of fluorine ions into hydroxyapatite by a pH cycling method. Journal of Materials Science: Materials in Medicine 16(5): 447-453.
- Rajesh AP, T Erik, JW Thomas (2008) Increased osteoblast density in the presence of novel calcium phosphate coated magnetic nanoparticles. Nanotechnology 19(26): 265101.
- Gupta TK (1991) In: Engineered Materials Handbook Ceramics and Glasses, ASM 4: 1150.
- May S. Freaga, Ahmed O Elzoghby (2018) Protein-inorganic Nanohybrids: A Potential Symbiosis in Tissue Engineering, Review article, Current Drug Targets 19(16): 1897-1904.
- Tomohiro Iwasaki, Ryo Nakatsuka, Kenya Murase, Hiroshige Takata, Hideya Nakamura, et al. (2013) Simple and Rapid Synthesis of Magnetite/Hydroxyapatite Composites for Hyperthermia Treatments via a Mechano chemical Route. International Journal of Molecular Sciences 14(5): 9365-9378.
- Oleksiy Kudaa, Nataliya Pinchuka, Liana Ivanchenkoa, Oleksandr Parkhomeya, Olena Sycha, et al. (2009) Effect of Fe3O4, Fe and Cu doping on magnetic properties and behaviour in physiological solution of biological hydroxyapatite/glass composites, JMPT. Journal of materials processing technology 209: 1960-1964.
- F Filho, REFQ Nogueira, MPF Grac, MA Valente, ASB Sombra, CC Silva (2008) Structural and mechanical study of the sintering effect in hydroxyapatite doped with iron oxide. Physica B 403: 3826-3829.
- Palcevskis A, Dindune S, Putic I, Balac R, Petrovic Dj Janackovic (2010) Microwave sintering improves the mechanical properties of biphasic calcium phosphates from hydroxyapatite microspheres produced from hydrothermal processing, Dj. Veljovic, E. Journal of Materials Science, vol. 45: 3175-3183.
- Youcai Liu, Hong Zhong, Lifeng Li, Chunjing Zhang (2010) Temperature dependence of magnetic property and photocatalytic activity of Fe3O4/hydroxyapatite nanoparticles, Materials Research Bulletin 45(12): 2036-2039.
- Chunjing Zhang, Shihui Si, Zhengpeng Yang (2015) Facile synthesis of Fe3O4/SiO2/molecularly imprinted hydroxyapatite nano composite and its enhanced photo catalytic degradation of target, contaminant, Separation and Purification Technology 143: 88-93.
- Zheng Peng Yang, Xue Yun Gong, Chun Jing Zhang (2010) Recyclable Fe3O4/Hydroxyapatite composite nanoparticles for photo catalytic applications. Chemical Engineering Journal 165(1): 117-121.
- Xie W, Zang X (2017) Covalent immobilization of lipase onto amino propyl-functionalized hydroxyapatite- encapsulated-γ-Fe2O3 nanoparticles: a magnetic biocatalyst for inter esterification of soybean oil. Food Chem 227: 397-403.
- Mandal S, Natarajan S (2015) Adsorption and catalytic degradation of organic dyes in water using ZnO/ZnxFe3-xO4 mixed oxides. J Environ Chem Eng 3(2): 1185-1193.
- M Elkady, H Shokry, H Hamad (2018) Microwave-assisted synthesis of magnetic hydroxyapatite for removal of heavy, metals from groundwater. Chem. Eng. Technology 41(3): 553-562.
- Itendra Kumar Sahoo, Monidipa Konar, Juhi Rath, Devendra Kumar, Harekrushna Sahoo (2019) Magnetic hydroxyapatite nanocomposite: Impact on eriochrome black-T removal and antibacterial activity, Journal of Molecular Liquids pp. 111596.

 Research Article
Research Article