Abbreviations: CVI: Chronic Venous Incompetence; GSV: Great Saphenous Vein; SSV: Small Saphenous Vein
Introduction
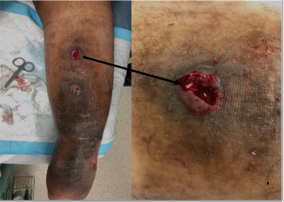
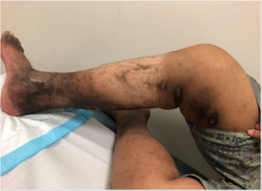
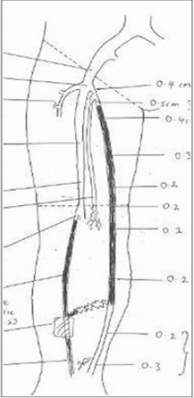
The VenaSealTM closure system (Medtronic, Minnesota, USA) is a medical tissue adhesive containing n-butyl-2 cyanoacrylate, injected endovenously to treat varicose veins and superficial chronic venous incompetence (CVI), resulting here in a new treatment –related complication. This 42-year-old Malay male underwent endovenous ablation of the right great saphenous vein (GSV) and small saphenous vein (SSV) in November 2017 with the device for CEAP 6 disease. He had no previous drug allergies. The procedure was uneventful but unfortunately he started developing multiple painless large ‘pustules’ with surrounding erythema along the treated GSV and SSV 2 weeks post-operatively. This was initially thought to be a phlebitic type reaction, which is common side -effect after this procedure [1]. The patient was treated with anti-inflammatories and antibiotics assuming an element of underlying infection. However, his infection markers were never raised. All the pustules eventually burst and to our surprise pieces of white glue casts were seen from each wound (Figure 1). Histology of the glue cast showed degenerative inflammatory debris [2]. Subsequently, more glue casts were extruded periodically over the next 6-12 months until all the wounds were healed completely (Figure 2). Surveillance ultra sound scan at 12 months showed that both GSV and SSV remained completely ablated (Figure 3). Patient remained asymptomatic from his treated CVI.
Discussion
Hypersensitive reaction to cyanoacrylate has been reported when used as topical skin adhesive in place of traditional sutures for post-surgical wounds [3] and as cosmetic adhesive, for example, fixation of wigs [4]. To the best knowledge of authors, this phenomenon associated with use of glue for treating varicose veins has never been reported in the literature. The authors believe this is likely to be a hypersensitivity reaction to cyanoacrylates rather than infection-based on the clinical presentation. In this patient, there were no significant adverse outcome in wound healing and the efficacy of the treatment apart from the post-inflammatory hyperpigmentation. Allergy reactions to cyanoacrylate skin adhesive can be managed by immediate removal of the adhesive. However, for VenaSeal, removal of the glue casts involves surgical intervention and therefore the implication may be more significant. This case has illustrated the natural history of hypersensitivity to cyanoacrylate may present when it is used for treatment of varicose veins. The senior author (TYT) no longer offers this option of venous ablation to patients with multiple drug allergies as he has found that a hypersensitivity reaction is more likely in these types of patients.
References
- Tang TY, Tiwari A (2018) The VenaSealTM Abnormal Red Skin Reaction: Looks Like but is not Phlebitis!. Eur J Vasc Endovasc Surg 55(6): 841.
- Tang TY (2018) Endovenous Cyanoacrylate Super-Glue to Treat Varicose Veins: How I Perform VenaSeal Ablation - Tips & Tricks Gained from Ablating Over 300 Truncal Saphenous Veins. Annals Vasc Med Surg 1(1): 1-5.
- Bowen C, Bidinger J, Hivnor C, Hoover A, Henning JS (2014) Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate. Cutis 94(4): 183-186.
- Sornakumar L, Shanmugasekar C, Rai R, Priya S (2013) Allergic Contact Dermatitis to Superglue. Int J of Trichology 5(1): 43-44.

 Case Report
Case Report