Introduction
Positron Emission Tomography (PET) is a multidisciplinary science. In contrast to CT and MRI, which are structural imaging techniques, PET is based on the characteristics of positron emitterslabeled radiopharmaceuticals and has been applied to study human pathophysiology in vivo non-invasively. In addition, PET has also been applied to monitor the efficacy of therapies, study drug pharmacokinetics (PK) and drug pharmacodynamics (PD). To design PET radiopharmaceuticals for brain neurotransmitters imaging, several factors has to be taken into consideration:
1) Binding Affinity: 0.01-1nM;
2) Binding Selectivity and Specificity: the higher the better;
3) Lipophilicity: Log P=1.5-4;
4) Specific Activity: the higher the better;
5) In vivo stability;
6) Uptake kinetics; and
7) Toxicity and Radiation Dosimetry
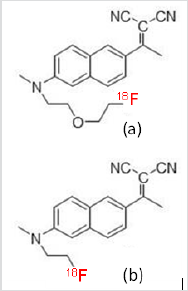
Alzheimer’s disease (AD) is one of the most frequent causes of death and disability worldwide and has a significant clinical and socio-economic impact. Although the precise cause of AD remains unclear, it is most likely due to multiple etiologies such as neuronal apoptosis, inflammatory responses, and alterations in various receptors and enzymes. Thus, several PET imaging agents that target multiple mechanisms such as various receptors, enzymes and β–amyloids have been developed [1,2] for monitoring the response of AD drug therapy non-invasively and facilitating AD drugs development. [18F]FDDNP (Figure 1b) was one of the first tau protein PET imaging radiopharmaceuticals for Alzheimer’s disease and chronic traumatic encephalopathy [3]. In order to increase its lipophilicity and consequently improve its brain uptake, we have synthesized its analog ([18F]FEONM, Figure 1a) and evaluated it as a potential tau protein imaging agent.
a. [18F]FEONM and
b. [18F]FDDNP
Figure 1: Structure modification of [18F]FDDNP will increase the lipophilicity and the new structure named [18F]FEONM. Therefore, the no Beta amyloid [18F]FDDNP has become an both Tau tangle and Beta amyloid uptake Alzheimer disease imaging agent [18F]FEONM.
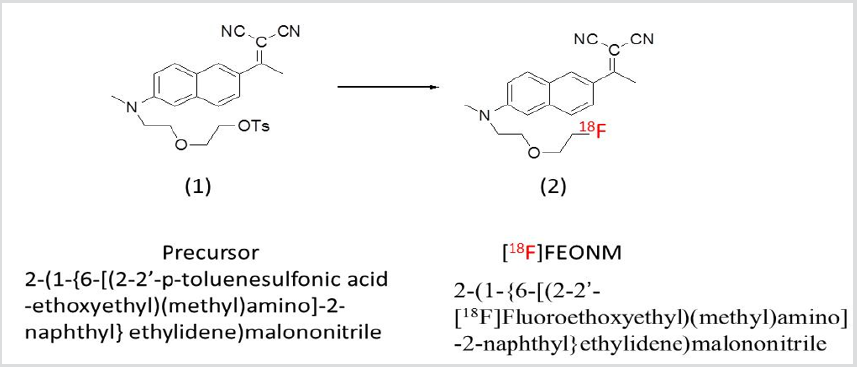
The [18F]FEONM (Figure 2, 2) was synthesized by nucleophilic fluorination of the corresponding tosyl- precursor (Figure 2, 1) in acetonitrile with K[18F]/K2.2.2 at 95 ℃ for 15min followed by purification with a semi-preparative HPLC (Fortis® Part No. FPH 100905, 5μm diphenyl, size: 250x10mm) and solid phase extraction gave product in 20-30% non-decay corrected yield (EOS) in a synthesis and purification time of 40min from EOB. The radiochemical purity of [18F]FEONM was determined using an analytical HPLC (Cogent C18 100A 5μm, e Series,150mmx4.6mm, Cat.No.78018-15P). The keystep of producing F-18 labeled PET radiopharmaceuticals online is radiofluorination. One of the best reactors for radiofluorination is made of carboxy glass [4]. In carboxy glass reactor, the reaction rate of [18F]FEONM will be a first order reaction in the beginning, with n order reaction at time ta to achieve highest yield and end at time tb, then gradually decompose with -m order reaction and end at time tc. Therefore, for the function of gap area (FG) [5] curve can be approached with Gauss distribution, Gauss or Welch apodization function. Since the solution of the integration form of Gaussian apodization function is error function, cannot be found an analytical form at its minimum area by calculus. However, the length of microfluidic plug flow reactor can be designed based on Welch apodization function with an analytical form as:
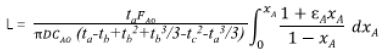
Where D: diameter, FA0: feed rate, CA0: [18F-] initial activity concentration, εA: stoichiometry, xA: conversion rate. In vitro studies showed that [18F]FEONM had higher lipophilicity than that of [18F]FDDNP (log = 2.20 ± 0.17 and 1.93 ± 0.10, respectively [5-7]) and the logP ratio of [18F]FEONM to [18F]FDDNP is 1.14. Thus, the lipophilicity of [18F]FDDNP increases as expected and it gets 38% higher. This is measured with shake-flask gold standard method which is lower than the HPLC measuring result [4,5]. The measured lipophilicity difference between these two methods is about one hundred times, which is very similar to Harmine, FE@SUPPY, DASB, WAY100635 and SNAP-7941 [8] also like the trending of Harmol and FET [8].
Micro PET imaging in Streptozotocin induced Tau tangle mouse model [9] showed that the brain hippocampus uptake of [18F]FEONM in Tau tangle brain hippocampus to cerebellum [18F] FEONM on a Tau tangle P301S/PS19 transgenic mouse model is 2.29 [5] while it is 1.78 and 1.51 in Beta amyloid Tg2576 transgenic mouse model and in a triple transgenic 3xTg mouse model which has both Tau tangle and Beta amyloid [10]. From the transgenic mouse model imaging study, we found that [18F]FEONM had uptake on both Tau tangle and Beta amyloid transgenic mouse which is different from that of [18F]FDDNP that showed no Beta amyloid transgenic mice uptake in brain hippocampus [11].
References
- Natsume O, Kabashima S, Nakazato J, Yamamoto Hanada K, Narita M, et al. (2017) Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): a randomized, double-blind, placebo-controlled trial. The Lancet 389(10066): 276-286.
- Bellach J, Schwarz V, Ahrens B, Trendelenburg V, Aksünger Ö, et al. (2017) Randomized placebo-controlled trial of hen’s egg consumption for primary prevention in infants. J Allergy Clin Immunol 139(5): 1591- 1599.
- Wei-Liang Tan J, Valerio C, Barnes EH, Turner PJ, Van Asperen PA, et al. (2017) A randomized trial of egg introduction from 4 months of age in infants at risk for egg allergy. J Allergy Clin Immunol 139(5): 1621-628.
- Palmer DJ, Sullivan TR, Gold MS, Prescott SL, Makrides M (2017) Randomized controlled trial of early regular egg intake to prevent egg allergy. J Allergy Clin Immunol 139(5): 1600-1607.
- Perkin MR, Logan K, Tseng A, Bunmi Raji, Salma Ayis, et al. (2016) Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. New England Journal of Medicine 374: 1733-1743.
- Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, et al. (2013) Early regular egg exposure in infants with eczema: A randomized controlled trial. J Allergy Clin Immunol 132(2): 387-392.
- Stuart EA, Bradshaw CP, Leaf PJ (2015) Assessing the Generalizability of Randomized Trial Results to Target Populations. Prevention science : the official journal of the Society for Prevention Research 16(3): 475-485.
- Någren K, Halldin C, Rinne J (2010) Radiopharmaceuticals for positron emission tomography investiga tions of Alzheimer’s disease. Eur J Nucl Med Mol Imaging 37(8): 1575-1593.
- Vallabhajosula S (2011) Positron emission tomography radiopharmaceuticals for imaging brain Beta-amyloid. Semin Nucl Med 41(4): 283-299.
- Barrio JR, Small GW, Wong KP, Huang SC, Liu J, et al. (2015) In vivo characterization of chronic traumatic encephalopathy using [F-18] FDDNP PET brain imaging. PNAS 112(16): E2039-E2047.
- Hamaker K, Blessing G, Nebeling B (1990) Computer aided synthesis (CAS) of no-carrier-added 2-[18F] fluoro-2-deoxy-D-glucose: an efficient automated system for the aminopolyether-supported nucleophilic fluorination. Appl Radiat Isot 41(1): 49-55.
- Hsu JP, Chen JT, Lin SH, Chu KY, Tu YH, et al. (2016) Radiofluorination process development and tau protein imaging studies of [F-18] FEONM. J Taiwan Inst Chem Eng 68: 119-129.
- Luurtsema G, Schuit RC, Takkenkamp K, Lubberink M, Hendrikse NH, et al. (2008) Peripheral metabolism of [18F] FDDNP and cerebral uptake of its labelled metabolites. Nucl Med Bio 35(8): 869-874.
- Chang KW, Chen CC, Lee SY, Wang HE (2009) Acute toxicity of two Alzheimer’s disease radiopharmaceuticals: FDDNP and IMPY. Drug and Chemical Toxicology 32(4): 429-437.
- Vraka C, Nics L, Wagner KH, Hacker M, Wadsak W, et al. (2017) LogP, a yesterday’s value? Nucl Med Bio 50: 1-10.
- Pradip KK (2015) Streptozotocin induced Alzheimer’s disease like changes and the underlying neural degeneration and regeneration mechanism. Neural Regen Res 10(7): 1050-1052.
- Kane AE, Shin S, Wong AA, Fertan E, Faustova NS, et al. (2018) Sex differences in healthspan predict lifespan in the 3xTg-AD mouse model of alzheimer’s disease. Front Aging Neurosci 10:172.
- Kuntner C, Kesner AL, Bauer M, Kremslehner R, Wanek T, et al. (2009) Limitations of small animal PET imaging with [18F] FDDNP and FDG for quantitative studies in a transgenic mouse model of Alzheimer’s disease. Mol Imaging Biol 11(4): 236-240.

 Short Communication
Short Communication