Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Almodóvar P1, González Hedström D,2, Jarama I1, Salamanca A1 and Inarejos García AM*1
Received:January 28, 2019; Published: February 07, 2019
*Corresponding author: Antonio Manuel Inarejos-García, Pharmactive Biotech Products, Madrid, Spain
DOI: 10.26717/BJSTR.2019.14.002519
The aim of the present study was to analyse and to compare the proteolytic enzyme actinidin content of fresh kiwifruit (Actinidina deliciosa) and the commercial kiwi extract KWD+®. The total protein extract was quantified by Bicinchoninic Acid Assay subjected to electrophoretic separation on an SDS-PAGE gel by “Coomassie brilliant blue” stain, the identification of Actinidin-A by immunoassay and the quantification with the corresponding purified standard. The actinidin concentration of KWD+® was about 5 fold higher than fresh kiwifruit (from 4.1 to 0.8mg of actinidin per grams of the sample). The standardization to actinidin in KWD+® allows to stablish a concrete dosage to improve the digestion of some proteins difficult to digest such as gluten, soy protein or beef muscle.
Keywords: Actinidina Deliciosa; Kiwifruit Extract; Actinidin; Proteolytic Enzyme; Gluten; Digestion
Kiwifruit (Actinidina Deliciosa) is an exceptional source of nutrients such as vitamin C, and other bioactive components related to its healthy effects such as metabolic health, digestion, antioxidant activity and immune function [1].One of the best-known properties of kiwi consumption is the improvement of protein digestion due to the presence of actinidin (EC 3.4.22.14), a proteolytic enzyme [2]. There are several In vitro and In vivo studies that show the proteolytic effect of actinidin from kiwifruit in different food proteins such as gluten, soy protein or beef muscle [3-6]. The aim of this research work was to study the actinidin content of a new kiwifruit extract, and to compare it with the whole kiwifruit.
The commercial kiwifruit extracts (KWD+®) provided by Pharmactive Biotech Products together with the fresh kiwifruit in powder form (which consists of a fresh kiwifruit freeze-dried), were stored in darkness at room temperature previous to analyses.
The fresh kiwifruit powder(KP) and KWD+® samples were previously prepared according to Martin (2016) to extract the protein components. Briefly, 500 mg of sample was subjected to a non-reductive lysis of the lipids by incubation for 16h at 4 °C in the presence of 2 mL of buffer. The resultant solution was centrifuged, and the soluble phase (supernatant that contains actinidin) was stored. The total protein extract was quantified by BCA (Bicinchoninic Acid Assay; Thermo Fisher Scientific, Massachusetts, USA), which was subjected to electrophoretic separation on an SDS-PAGE gel (12%). The total protein profile was performed by “Coomassie brilliant blue” stain (BioRad, California, USA) and the identification of Actinidin-A by immunoassay using a polyclonal antibody anti-actinidin A (anti-Cp2, Agrisera antibodies, Vännäs, Sweden), and visualised with an anti-rabbit peroxidase as secondary antibody. The quantitative analysis of Actinidin was performed with the purified standard Actinidin (KiwiEnzyme Ltd, New Zealand), the concentrations employed were between 1 to 200 µg/µL. In addition, a pattern of BSA concentrations was performed, with a range between 0.5 at 40 µg/µL. The quantification of Actinidin and total protein content was performed by digital images on protein gels and blots using the processing software Fiji (https://fiji.sc/).
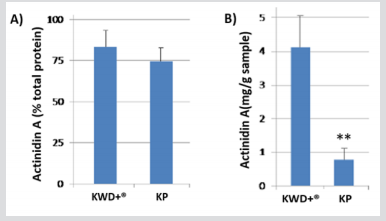
Coomassie brilliant blue staining of the KWD+® and KP samples have a major band between 30-35 KDa, which was identified as Actinidin A using immunoblot techniques (antibodies reactive against Actinidin A), with more than 70% of its total protein content as can be seen in Figure 1A Apart from actinidin A, other three bands of lower molecular weight were observed, which contribute 18-28 % to the total protein content of each sample (data not shown). Nevertheless, KWD+® sample showed about 5-fold higher levels of Actinidin A compared to the fresh kiwifruit (KP) samples (Figure 1B) P≤ 0.01. Kiwifruit has a low protein content, being the actinidin more than 50 % of the total protein content, which agrees with the results obtained by other authors [7-11]; in the case of the new KWD+®, the actinidin content (in percentage) represent more than 80% (82±9 %) of the total protein, and KP was slightly lower (74±5 % Figure 1A). However, the actinidin content in KWD+® (4.1±0.9 mg actinidin/g sample) was significantly higher compared to KP (0.8±0.3 mg Actinidin/ g of sample; (Figure 1B)).
Figure 1: Actinidin A concentration based on A) total protein content (%) and B) Actinidin A content (mg/g sample) in KWD® and KP samples
The extraction processing technology may increase the actinidin concentration at least 5-fold in the KWD+® extract compared to the fresh kiwifruit, and therefore, the development of a new kiwifruit extract standardized to actinidin may improve the digestion of specific proteins difficult to digest using an adequate dosage


