Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Paolo Romita*1, Domenico Bonamonte1, Grazia Ettorre1, Monica Corazza2, Alessandro Borghi2 and Caterina Foti1
Received: January 11, 2019; Published: January 22, 2019
*Corresponding author: Paolo Romita, Department of Biomedical Science and Human Oncology, Bari, Italy
DOI: 10.26717/BJSTR.2019.13.002396
Acne is a chronic disease that generally requires long-lasting therapy with topical drugs. The potential for contact sensitization of topical anti-acne drugs is low [1], but the incidence of ACD may be underestimated, since several cases may not have been published, or have been misdiagnosed as irritant contact dermatitis.
Keywords: Allergic Contact Dermatitis; Acne Vulgaris; Angioedema; Benzoyl Peroxide; Case Report; Clindamycin; Patch Tests; Urticaria
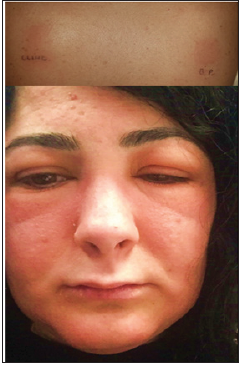
A 22-year-old non-atopic woman presented to the Emergency Unit with a severe erythematous-oedematous eruption on her face (Figure 1) associated with severe itching and burning. In particular, severe eyelid oedema caused almost complete palpebral fissure closure. In this case, suspecting urticaria/angioedema, the casualties department treated her with intravenous injections of betamethasone and chlorphenamine maleate, prescribing scaling doses of betamethasone in the following days. After the resolution of the dermatitis she came to our attention referring that the eruption had appeared about 1 month after the start of topical therapy with Duac® 5% gel (clindamycin 1% and benzoyl peroxide 5% ) prescribed by her physician to treat papulo-pustular acne of moderate severity. Hence, patch tests with the S.I.D.A.P.A. (Italian Society of Allergological, Occupational and Environmental Dermatology) baseline series (Euro medical, Calolziocorte, LC, Italy) and all the topical products used by the patient (tested ‘as is’) were performed. Patch tests with Al Test® (Euro medical) on Scanpor® Tape (Norgesplaster, Vennesla, Norway) were applied on the back and left in occlusion for 2 days. Readings were performed at day (D)2 and D4 following ESCD guidelines [2], showing positive reactions to Duac 5%® gel (++). Subsequently, patch tests were performed with the ingredients of Duac 5%® gel at the appropriate concentrations, and positive (++) (Figure 1) results were obtained to clindamycin 1% pet. and benzoyl peroxide (BPO) 1% pet. ROAT with clindamycin 1% pet and BPO 1% were performed on the volar aspect of the forearm (5 × 5cm applications twice daily), showing a positive reaction after 3 applications to the former, and 1 application to the latter.
Figure 1: Positive patch tests to clindamycin 1% pet. and benzoyl peroxide 1% pet. at D4, and the facial eruption resembling urticaria/angioedema.
We report a case of ACD to topical clindamycin and BPO in a patient affected by acne vulgaris. Clindamycin was first reported as sensitizing in 1978 [3] and since then, few cases of ACD have been described [4-9]. BPO is a common contact sensitizer when used to treat leg ulcers [10,11], while it is rarely reported as sensitizing in acne patients, even if the incidence may be underestimated [12]. Moreover, topical preparations with BPO are well-known to be irritant, so the first symptoms of ACD may be mistakenly ignored. Risk factors for ACD in acne patients include characteristic skin barrier injuries [1] and irritation caused by retinoids or BPO. In [13], the authors suggested that irritant contact dermatitis, that is typical in the first weeks of treatment, could enhance the risk of ACD to topical anti-acne drugs. To our knowledge, this is the first case of concomitant sensitization to clindamycin and BPO caused by topical anti-acne treatment. Differential diagnosis included airborne contact allergy [14], contact allergy to cosmetics caused by rarely sensitizing substances [15-18] and photoallergic contact dermatitis. Anyway, the history and the results of the patch tests were diriment. We wish to underline the importance of bearing in mind that skin flare during topical anti-acne therapies could be related to ACD, and it is therefore essential to perform patch tests with the baseline series and with the topical products used by the patient, as well as all their ingredients. Continued usage may result in severe reactions, as in our case, that may resemble other dermatological diseases and so mislead physicians, delaying the correct diagnosis.


