Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Folusakin Ayoade*
Received:January 01, 2019; Published:January 18, 2019
*Corresponding author: Folusakin Ayoade, Department of Medicine, Division of Infectious diseases,1120 NW 14TH Street, Miami, FL 33136, USA
DOI: 10.26717/BJSTR.2019.13.002378
Toxoplasmosis is one of the commonest causes of morbidity and mortality in severely compromised HIV patients and prophylaxis is often recommended. Going by several United States management guidelines, the CD4 lymphocyte cut off to start toxoplasmosis prophylaxis is below 100 cells/μL. This threshold may need to be raised to 200 cells/μL as extensive toxoplasmic encephalitis is increasingly described in HIV patients with CD4 lymphocyte counts between 100 and 200 cells/μL. The suggested adjustment in the CD4 count threshold will offer protection to more at-risk population and bring the US guidelines in harmony with the European guidelines
Keywords: Toxoplasmosis; Prophylaxis; CD4; HIV; Guidelines
Toxoplasmosis is the most common infectious cause of focal brain lesions in human immunodeficiency virus (HIV) infection and it is often associated with significant morbidity and mortality [1]. Toxoplasma gondii, the causative agent is an intracellular protozoan parasite with worldwide distribution. The local disease incidence however is closely linked to toxoplasma seropositivity in any given population [2]. Seropositivity of toxoplasmosis in HIV infected patients has been shown to be high and prophylaxis is generally recommended for immunocompromised HIV patients who have positive toxoplasma immunoglobulin G [3]. Toxoplasmosis in HIV infected patients is commonly seen when the CD4 lymphocyte count falls below 100 cells/μL and in many patients, CD4 counts are often below 50 cells/μL [4]. Not every HIV patient with reactivation toxoplasmosis however presents with very low CD4 counts. While approximately 75% of patients will have CD4 counts less than 100 cells/μL at the time of presentation, 90% or more will have CD4 counts below 200 cells/μL.
Table 1: Adpated from NIH/HIVMA/IDSA (US guidelines) on preventing Toxoplasma gondi encephalitis in HIV positive adults and adolescents.
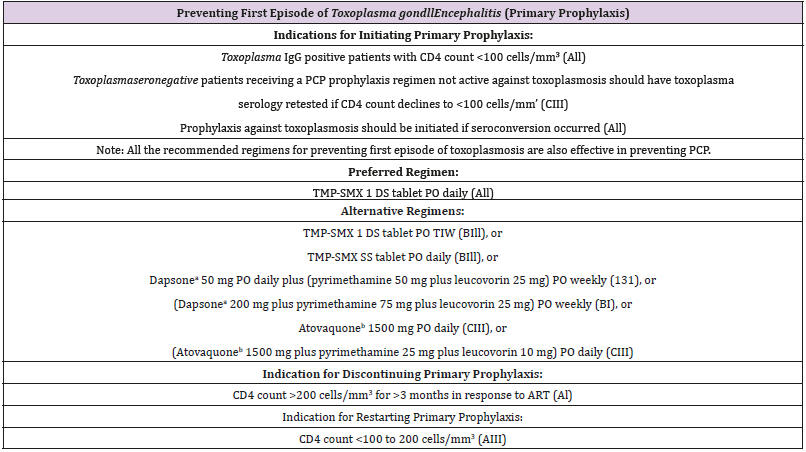
Recommendations from major United States health organizations including the Centers for Disease Control and Prevention, the National Institutes of Health (NIH), and the HIV Medicine Association of the Infectious Diseases Society of America (HIVMA) [5] suggest providing toxoplasmosis prophylaxis in toxoplasma seropositive individuals only when the CD4 lymphocyte count falls below 100 cells/μL (Table 1). This recommendation leaves as many as 25% of toxoplasma seropositive HIV patients at risk who can develop toxoplasmic encephalitis at CD4 count above 100 cells/μL. In contrast to the American guidelines, the European guidelines [6] favor starting toxoplasmosis prophylaxis when the CD4 count falls below 200 cells/μL (Table 2). Some observations support the possibility of extensive cerebral toxoplasmosis at higher CD4 counts (above 100 cells/μL) either in newly diagnosed toxoplasma seropositive HIV patients or those already established on antiretroviral therapy [7,8].
Table 2: Adapted from the European AIDS Clinical Society Guidelines on primary prophylaxis of opportunistic Infections in HIV.
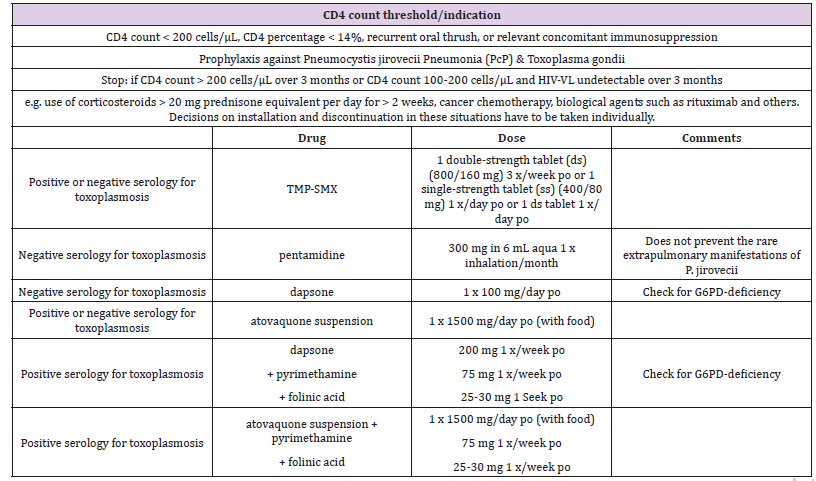
HIV patients with CD4 counts between 100 and 200 cells/ μL have substantially increased risk of developing toxoplasmic encephalitis than those with CD4 counts greater than 400 cells/ μL [9]. This group of patients with relatively higher CD4 counts (between 100 and 200 cells/μL) would not have been started on definitive toxoplasmosis prophylaxis going by the United States (US) guidelines. It is reasonable to say that effective antiretroviral therapy and widespread pneumocystis prophylaxis in HIV patients who have CD4 counts less than 200 cells/μL have minimized toxoplasmosis reactivation. In all fairness, one may argue that at CD4 counts between 100 and 200 cells/μL, most HIV patients will typically be on some form of pneumocystis prophylaxis which will also be protective against toxoplasmosis [10,11]. Going by this argument, in the setting of appropriate adherence to antiretroviral therapy, definitive need for additional toxoplasmosis prophylaxis may be less important. The problem with this viewpoint is that US providers who abide strictly by the US guidelines may not initiating toxoplasma prophylaxis until CD4 counts falls well below 100 cells/ μL.
In the same vein, in patients allergic to sulfamethoxazole/ trimethoprim, the most prescribed and most effective prophylactic agent for pneumocystis [12], additional agents such as pyrimethamine and atovaquone is often needed to provide adequate prophylaxis against both pneumocystis and toxoplasma infections [6,13,14]. Using the current US guidelines, adequate prophylaxis may therefore be compromised when the CD4 counts falls between 100 - 200 cells/μL for this cohort. Clinical symptoms of opportunistic infections including toxoplasmosis have been known to develop or become unmasked shortly after initiation of antiretroviral therapy. This is partly related to the restoration of the immune system in a previously immunosuppressed patient [15]. In addition, appropriate immune reconstitution may be delayed or inadequate in some patients on HIV treatment, due to erratic compliance with antiretroviral therapy or due to the development of drug resistance. All these factors suggest HIV viremia and suboptimal CD4 counts are good possibilities even in the treatment experienced patients.
The need for prophylaxis against appropriate opportunistic infections, based on the degree of immunosuppression is therefore still justified [16]. My suggestion is that the US guidelines should be reviewed and possibly amended by changing the threshold for initiating toxoplasmosis prophylaxis from 100 cells/μL to 200 cells/ μL [9]. The updated NIH and HIVMA guidelines on opportunistic infections in HIV patients underscore the fact that the risks of acquiring toxoplasma encephalitis at CD4 counts between 100-200 cells/μL have not been rigorously studied [5]. Is it possible that the current US toxoplasmosis prophylaxis recommendation may be too narrow or restrictive? Is it possible that we may be leaving a fair number of HIV patients from areas of high toxoplasma seropositivity at risk of disease reactivation? I will also suggest that more research be directed at this group of HIV patients with CD4 counts between 100-200 cells/μL with respect to toxoplasmosis prophylaxis. In the meantime, it is probably reasonable to keep the message simple by harmonizing the recommendations for primary prophylaxis for both pneumocystis and toxoplasmosis at the same CD4 cut off. This will provide prophylactic coverage to more patients at risk including those with sulfamethoxazole/trimethoprim allergy and avoid a false sense of security for providers.


