Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nichollas Guimarães Jaques1, Andreas Ries2, Ingridy Dayane dos Santos Silva1, Marcus Vinicius Lia Fook2 and Renate Maria Ramos Wellen*1,2
Received:December 18, 2018; Published: January 09, 2019
*Corresponding author: Renate Maria Ramos Wellen, Materials Engineering Department, Brazil
DOI: 10.26717/BJSTR.2019.12.002320
Non-isothermal melt and cold crystallization kinetics of poly(3-hydroxybutyrate) (PHB) and titanium dioxide (PHB/ Tio2) compounds, with Tio2 content ranging from 1-10% per weight, were investigated at different cooling/heating rates (5, 7.5, 10, 15, 20 and 30 °C/min) using Differential Scanning Calorimetry (DSC). Pseudo-Avrami model as well as Friedman’s isoconversional methods were used for analyzing the non-isothermal crystallization process. The results showed that the models provide a fair description of the non-isothermal crystallization processes, provided that the model parameters n and ln(K) were determined for the specific condition of interest. It was found that Tio2 accelerates the crystallization. Friedman’s effective activation energy reveals that melt and crystallization processes follow different mechanisms.
Keywords: PHB; Tio2; Crystallization Kinetics; Friedman Activation Energy
Poly(3-hydroxybutyrate) (PHB) is a very promising, completely biodegradable and biocompatible polymer, available from renewable sources. It has been used in a number of industrial applications, including packing, food containers, prosthesis, suture threads, devices for controlled drug release, containers for plant germination, blankets for pesticides release, etc. The crystallization and melting behavior of PHB has been studied with the objective to control its microstructure, since the physical properties of the polymer depend on the crystallization conditions during processing, and there is still much to be learned about the way it responds to thermal treatment [1-7]. Our research group is involved in the study of non-isothermal crystallization of PHB by means of DSC [8-10]. For a number of reasons, polymer materials, including PHB, are modified with natural or synthetic fillers. In this work we added Tio2 into a PHB matrix, with concentrations ranging from 1% to 10% per weight.
Tio2 is a widely used white pigment because of its brightness and very high refractive index. Important application areas of Tio2 are paints, varnishes, paper, plastics, rubber, cosmetic products and foodstuffs. Several studies have been performed concerning the effect of Tio2 into polymer matrices [10-15]. The present contribution is concerned with PHB/ Tio2 compounds prepared by melt mixing in a laboratory internal mixer; five compositions were produced with Tio2 content ranging from 1% to 10% by weight. The non-isothermal melt and cold crystallization processes were investigated by DSC applying heating/cooling/reheating cycles (six different heating/cooling rates). Kinetic results were described using the Pseudo-Avrami model. Friedman’s isoconversional methodology [16] was employed to estimate the activation energies as functions of the relative crystallinity.
PHB was supplied by PHB Industrial SA (Brazil) and was used without any further treatment. Tio2 was purchased from Evonik Degussa Co. (manufacturer’s specification P25), with a surface area of 50m2/g and a 75:25 anatase: rutile ratio. According to the manufacturer, the mean crystal sizes of the anatase and rutile phases are approximately 25 and 94nm.
PHB compounds containing 1, 2, 4 and 10% per weight Tio2 were prepared in a Haake Rheomix 600 laboratory internal mixer fitted with high intensity rotors, with the chamber temperature kept at 180 °C. The nominal rotor speed was set at 60rpm and the material was processed for 10min. Equally neat PHB was processed in the mixer. All samples were stored in a desiccator over silica gel overnight prior to processing and DSC analyses.
Non isothermal melt and cold crystallization tests of PHB and PHB/ Tio2 compounds carried out in a TA Instruments DSC Q20 differential scanning calorirmeter, under a nitrogen flow of 50ml/ min. Samples weighting between 4 and 6mg were heated to 190 °C and held this temperature for 3min; the melt was cooled to 20 °C and re-heated to 190 °C at predefined constant rates (5, 7.5, 10, 15, 20, and 30 °C/min). The heat flow was recorded as function of temperature and time and the exothermal (crystallization) peaks were analyzed.
The crystallization half time (t1/2) is defined as the time interval from the onset of crystallization until 50% of the total relative crystallinity is reached. This parameter may be used to judge the influence of Tio2 filler on the average crystallization rate during the first half of the process. Figure 1 shows t1/2 as function of the heating/cooling rate for the different compounds tested. At moderately low heating or cooling rates the half crystallization time diminishes with the rate that becomes practically independent of it at above 10 °C/min. The addition of filler reduces the half crystallization time for melt crystallization but has little effect on cold crystallization. These findings suggest that above certain temperature the filler accelerates crystal growth, a situation that occurs in melt crystallization at low cooling rates. Below that temperature the filler has little effect on crystallization rate, due perhaps to reduced polymer chain mobility; this happens for melt crystallization at high cooling rates and cold crystallization at any heating rate.
Figure 1: Crystallization half time of neat PHB and PHB/ Tio2 compounds as a function of the heating/cooling rate, considering melt and crystallization processes. (Data points only available where crystallization peaks were observed).
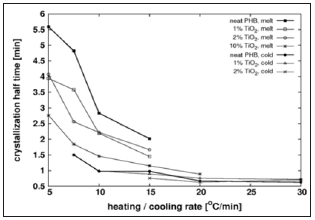
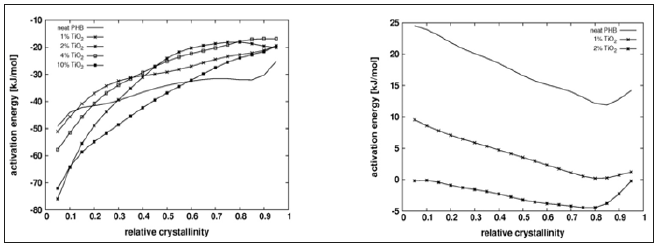
The onset of crystallization peak cannot be determined with precision by DSC, which limits the significance of the crystallization half time. Conversion rate versus relative crystallinity plots are presented in Figure 2 for the lowest cooling/heating rate tested, showing the acceletating effect of Tio2 on both, melt and cold. The plots show also the higher asymmetry of the peaks for melt crystallization compared with those for cold crystallization. Figure 3 shows the apparent activation energy estimated by Friedman’s method versus the relative crystallinity for melt and cold crystallization. Activation energy for cold crystallization decreases with conversion and shows a significant dependence on it, while the opposite is verified for melt crystallization: activation energy slightly increases and is fairly independent of conversion, suggesting that different mechanisms govern the two processes. Addition of Tio2 to the PHB matrix reduces the apparent activation energy for cold crystallization, an observation consistent with the increase in crystallization rate of the compounds, compared with the neat matrix as shown in Figure 2. However, full agreement between activation energy and conversion rate cannot be expected, as different compounds crystallize at different temperatures. The negative activation energies observed are unphysical and may result from the treatment of a complex multi-step process as a single step reaction with an Arrhenius type temperature dependence [17,18].
Figure 2: Conversion rate versus relative crystallinity for neat PHB and PHB/Tio2 compounds. Left: Melt crystallization at cooling rate 5 °C/min. Right: Cold crystallization at heating rate 15 °C/min, for Tio2 contents higher than 2%, no cold crystallization peaks could be observed in the DSC.
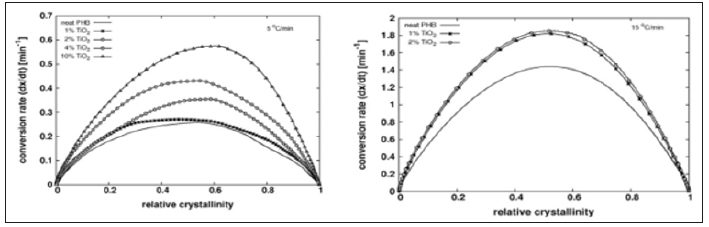
Figure 3: Dependence of the apparent activation energy on the relative crystallinity (x) for neat PHB and PHB/Tio2 compounds. Left: Melt crystallization; Right: Cold crystallization.
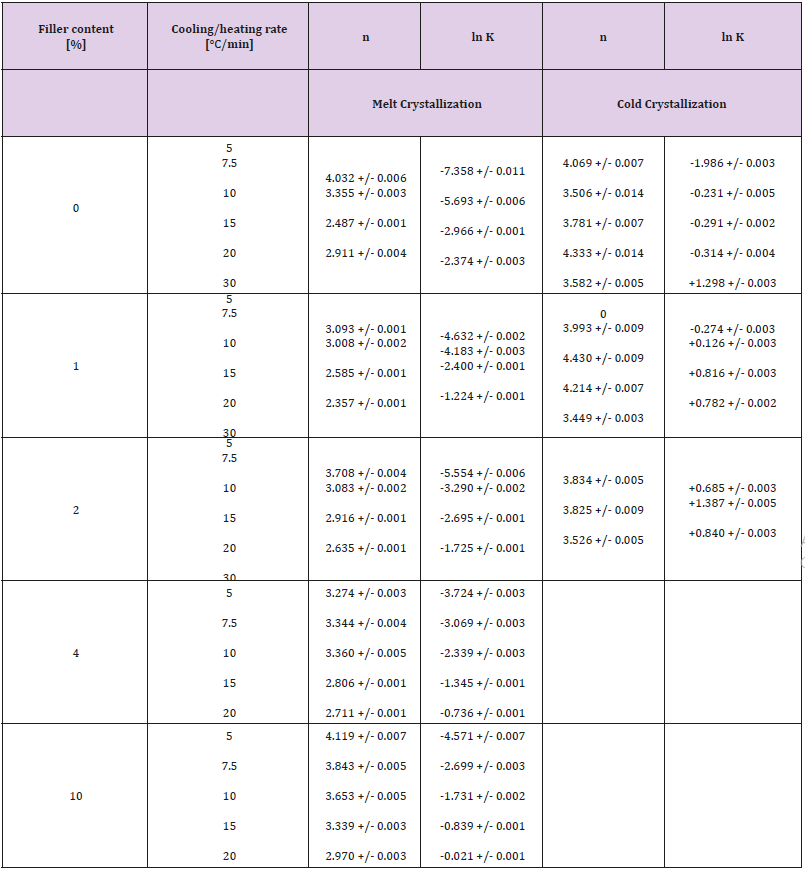
Pseudo-Avrami parameters were estimated by linear regression plotting ln[-ln(1-x)] versus ln(t), using only data points within the relative crystallinity interval 0.05 ≤ x ≤ 0.95. Numerical values of the parameters ln (Kp) and np are given in Table 1. The small uncertainty in the parameters recommends the model for data correlation of existing experimental data.
Table 1: Pseudo-Avrami parameters obtained by curve fitting procedure for melt and cold crystallization events
The Pseudo-Avrami model turned out to be suitable for a description of the non-isothermal crystallization kinetics of PHB and PHB/Tio2 compounds, this statement holds for both, melt and cold crystallization events. It was found that Tio2 nanoparticles accelerate both, melt and cold crystallization. Friedman’s effective activation energy reflects the different nature of the melt and cold crystallization mechanism.
Authors would like to thank PHB Industrial SA (Brazil) for kindly supplying PHB resin. Financial support from CNPq/Brazil is acknowledged (grant numbers PIBIC/CNPq/UFPB 10657 and PIBIC/CNPq/UFPB 10658). AR thanks CAPES for his post-doctoral fellowship.


