Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Helei Li1, Wei Tian1, Guangzhou Song1, Mengyao Ding1, Honggen Yi1, Yin Yin2, Fenglin Dong3 and Jiannan Wang*1
Received: December 19, 2018; Published: January 04, 2019
*Corresponding author: Jiannan Wang, National Engineering Laboratory for Modern Silk, China
DOI: 10.26717/BJSTR.2019.12.002310
Silk fiber is a natural protein with high purity, and can be easily processed into films, mesh and porous materials, which possesses good cytocompatibility and biodegradability, especially excellent water absorption and breathable. Base on the satisfactory properties, we prepared regenerated silk fibroin materials using neutral salt solvents dissolution with CaCl2, Ca(NO3)2 and LiBr, respectively (named SF-CaCl2, SF-LiBr and SF-Ca(NO3)2, and investigated platelet adhesion ability to evaluate driving force of hemostasis. Results showed that platelet adhesion rate and platelet activity on the calcium salt dissolved silk fibroin film surfaces were significantly higher that on the SF-LiBr film. SEM images showed that platelets tended to aggregate on the SF-CaCl2 and SF-Ca(NO3)2 films.
Keywords: Silk Fibroin; Films and Sponges; Platelet Adhesion Rate; Platelet Activity
Silk has rich sources and high purity, is composed of silk fiber core layer (silk fibroin) and sericin wrapped outer layer. Silk fibroin can be easily processed into various morphological materials, such as nanofibers, films, sponges, gels or nanoparticles [1-5], and has been widely studied for applications in extracellular matrix, tissue engineering scaffolds and drug delivery carriers, owing to its excellent biocompatibility and biodegradability [1-11]. On the other hand, silk fibroin contains more hydrophilic amino acids, such as serine, Tyrosine, glutamic acid, aspartic acid and lysine [12-14], endowing silk fibroin fast water absorption. On the bleeding wounds, rapid water absorption of a material is beneficial for improving blood viscosity, and promoting platelet activation and aggregation, thus accelerating clotting. Otherwise, the functional groups -COOH from glutamic acid or aspartic acid, and -OH from serine or Tyrosine can interact with Ca2+ by coordination bonds and electrostatic interaction [15,16]. In the coagulation mechanism, it is well known that Ca2+ is a key clotting factors, which interacts with platelet surface phospholipids to activate thrombin, and then digest fibrinogen into fibrin, resulting in coagulation formation. Therefore, silk fibroin protein is worth considering for the development of hemostatic materials. Platelets are the drivers in cascade pathway for blood clotting. In the present paper, we explored platelet adhesion and aggregation activity on regenerated silk fibroin materials prepared by different dissolution mechanisms, expecting to provide valuable guidance for the development of silk fibroin blood contact materials.
Raw silk was boiled in Na2CO3 solution three times to remove sericin, as described previously [9]. After degumming, silk fiber was dissolved in the following three neutral salt solvents (1) a ternary solvent CaCl2·CH3CH2OH·H2O (mole ratio, 1:2:8) at 70°C, or (2) 9.3 M of LiBr at 60°C or (3) molten Ca(NO3)2 at 85 °C, and stirred until completely dissolved. After dialyzing against deionized water, the mixed solution was concentrated and filtered to obtain silk fibroin aqueous solution (5%, w:w).
5% of silk fibroin aqueous solution were mixed with polyethylene glycol diglycidyl ether (Sigma, USA) (1.0:0.8, w/w) and stirred to ensure entirely mixing. The thin films of silk fibroin were prepared by casting the mixed solution onto polyethylene dishes and air-dried at room temperature. The sponges of silk fibroin were prepared by freeze-dried at -80°C. Samples were impregnated in deionized water at 4°C for 72 h to remove unreacted residues, and then cut and put into 48-well cell culture plates or 2 ml (0.5×2.0mm) centrifuge tubes, finally, were sterilized with gamma irradiation. Regenerated silk fibroin materials were named to SF-CaCl2, SF-LiBr, SF-Ca(NO3)2, respectively. Calcium alginate was used as a control.
Samples (in quintuplicate for sponges, in triplicate for films) were equilibrated with normal saline at 3°C for 2 h, then was added 1 mL (in 48-well plate chamber for static adhesion) or 1.5 mL (in 2-mL tube for dynamic adhesion) fresh platelet-rich plasma (PRP) at a density of 5×105 cells/mL (N1) and incubated at 37°C for 3 h. The PRP was obtained by the method described in the previous literature [17,18]. The numbers of platelets remaining in PRP were ounted using a cell counter (N2). The platelet adhesion rate was calculated using the following equation, all samples were analyzed in quintuplicate. Following rinsing, the sponges from each well or tube was used for platelet activity analysis, and the films for scanning electron microscopy (SEM) observation.
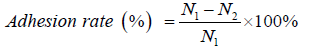
Platelet activity was determinated by methyl thiazolyl tetrazolium (MTT) method, as described previously [9,19]. Samples were added with MTT at working concentration 50 mg/mL and incubated at 37°C for 4-6 h. After removing the supernatant, 1 or 1.5 mL hydrochloric acid isopropyl alcohol was used to dissolve the purple formazan crystals. The absorbance of the supernatant was recored at 490 nm using a Synergy HT microplate reader (BIO-TEK, USA). All samples were analyzed in triplicate.
Samples were fixed in 2.5% glutaraldehyde for 2h and dehydrated using an ethanol/water gradient (25, 50, 75, and 100%). After air-drying in a fume hood and gold coating, platelet adhesion to each sample surface was observed on an S-4800 scanning electron microscope (SEM; Hitachi, Tokyo, Japan).
Data are presented as mean ± standard deviation of the mean. Comparisons of means were performed using one-way analysis of variance (ANOVA), followed by the independent sample t-test using SPSS 17.0 statistical software. Statistical significance was set at p< 0.05.
The platelet adhesion on the silk fibroin film surfaces is shown as Figure 1 Platelet adhesion to SF-CaCl2 and SF-Ca(NO3)2 was significantly more than to SF-LiBr. Ca2+, a proved coagulation factor in cascade coagulation pathways, interacts with platelet surface phospholipids and activates thrombin to digest fibrinogen into fibrin, resulting in coagulation formation. There is a large amount of functional groups -COOH, -NH2 and -OH distributed on silk fibroin chain, which can interact with Ca2+ by electrostatic interaction and coordination bonds. After dissolution by CaCl2 and Ca(NO3)2, there was a small amount of chelated Ca2+ remaining in silk fibroin films. So, platelet adhesion was greater on the calcium salt dissolved silk fibroin film surfaces. From SEM images, it can be seen most platelets on SF-CaCl2 and SF-Ca(NO3)2 films were activated, extended microfilaments and microtubules, and displayed high aggregation activity.
As shown in Figure 2 the platelet adhesion rate on SF-Ca(NO3)2 sponge (33%) was similar to that on SF-CaCl2 sponge (39%), and both of them was higher than that on SF-LiBr sponge (24%), owing to chelated Ca2+ in calcium salt dissolved silk fibroin. The platelet adhesion rate on calcium alginate (50%) was the highest because of a large amount of Ca2+ distributed in calcium alginate materials. Under static condition, the platelet adhesion rate on SFLiBr sponge was significantly lower than calcium alginate, but there was no significant difference between the calcium salt dissolved silk fibroin and calcium alginate. Dynamic platelet adhesion was slightly improved.
Platelets act the most important role in promoting blood coagulation, the strength of which is dependent on the platelet activity. As shown in Figure 3 platelet activities on SF-CaCl2 and SF-Ca(NO3)2 sponges were significantly higher compared with that on SF-LiBr sponge under whether static condition or dynamic condition, but lower than that of calcium alginate material. The results were consistent with the platelet adhesion rates.
Regenerated silk fibroin materials prepared by different dissolution strategies showed influences on suitable application. When using calcium salt dissolution to dissolve silk fibers, a small amount of Ca2+ chelated with silk fibroin molecules and remained in the silk fibroin after dialysis. It was a preferred method to prepare silk fibroin materials for the development of hemostatic materials, but not feasible to prepare anticoagulant materials, such as artificial blood vessels, intravascular stents or cardiac valves. The study of the paper provided a guidance for the applications of silk fibroin in blood contact materials.
This work was supported by National Natural Science Foundation of China [Nos 51473108, 51873141], Natural Science Foundation of Jiangsu Province of China [No BK20181192] and College Natural Science Research Project of Jiangsu Province of China [No 18KJA540001].


