Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sohair R Fahmy*, Khadiga Gaafar, Dawlat A Sayed and Huda Nageeb
Received: December 12, 2018; Published: January 03, 2019
*Corresponding author: Sohair R Fahmy, Department of Zoology, Faculty of Science, Egypt
DOI: 10.26717/BJSTR.2019.12.002302
Background: Chronic Kidney Disease (CKD) is a worldwide health threat. The Pyramids of Egypt are one of the world’s most amazing achievements. Thereby, the present study aims to investigate the ameliorative efficacy of housing Unilateral Nephrectomized (UNx) rats inside model of the great Egyptian pyramid.
Results: Housing UNx rats for 24 and 48h inside the Egyptian pyramid model induced significant increase in the weight of the right kidney, serum sodium and calcium levels and potassium ions excretion in urine. Creatinine clearance increased significantly after housing UNx rats inside the Egyptian pyramid model following 48 h. However, housing of rats inside the Egyptian pyramids for 24 and 48h ameliorate kidney function markers (proteinuria, urea and uric acid) and restore their levels towards the control values. In addition, levels of the reinin, angiotensin and aldosterone hormones significantly increased after housing UNx rats inside the Egyptian pyramid for 24 and 48h.
Conclusion: The present study showed that housing nephrectomized rats inside model of the Egyptian pyramid may be a potential new strategy prevents CKD following renal tumour resection. Also, the current study suggested that pyramid environment plays an important role in ameliorating the serious effects of nephrectomy by enhancing kidney function.
Keywords: Egyptian Pyramid Models; Unilateral Nephrectomized Rats; Kidney Functions; Renin-Angiotensin-Aldosterone System; Rats
Nephrectomy (Nx) is a novel intervention having first been introduced for the treatment of renal cell carcinoma in 1969 [1]. Although surgical resection remains the standard of care in the treatment of renal carcinoma, Nx is also a recognized risk factor for developing Chronic Kidney Disease (CKD) [2,3]. Indeed, nephrectomy is a widely used model to study the mechanisms leading to renal damage in CKD [4]. The intra-renal vasculature is the most affected structure, preventing an appropriate blood flow, fаvoring the glomerular sclerosis [5]. CKD will certainly lead to the progression of Chronic Renal Failure (CRF) due to gradual sclerosis of renal tissue. In addition, nephrons are gradually lost in renal failure. Remaining nephrons are hypertrophied to compensate for the loss of renal function, and hyperfiltration in these remaining nephrons leads to further nephron loss [6].
Models with the same dimensions of the great Egyptian pyramid of Giza are believed to generate, transform and transmit energy when aligned on a true north south axis [7]. The energy generated inside these models is of the electromagnetic spectrum and other forms or degrees of the so-called universal energy [8]. There is valid and important relationship between electromagnetic energy and the human body [9]. Human body flourishes in an electro-chemical environment, which is activated by its response to electromagnetic energy [10]. Razumov et al. [11] reported that electromagnetic waves induced a marked hemodynamic action in kidney via stimulation of the circulation of the intermediary zone with an effect of redistribution of the intra-renal blood flow. It was reported that the energy field developed within the pyramid may increase the Glomerular Filtration Rate (GFR) by increase in the renal blood flow following unilateral nephrectomy for 7 and 14 days [12]. The present study postulated that early preventing renal sclerosis following unilateral nephrectomy in rats may be achieved through improvement of the rheological properties of the blood under the effect of electromagnetic spectrum of the pyramid model that could delay the progression of the CRF. Thereby, the present study aims to investigate the ameliorative efficacy of housing Unilateral Nephrectomized (UNx) rats inside model of the great Egyptian pyramid.
Healthy male Wistar albino rats (Rattus norvegicus), weighing 180-200g, were used. The animals were obtained from the National Research Center (NRC), Egypt. The rats were kept for one week before the commencement of the experiment for acclimatization. Animals were grouped and housed in polypropylene cages (five animals per cage) in air conditioned room at temperature of 25 ± 1 oC and under natural day and night cycle. Animals were feed standard chow pellets and drinking water.
The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) (CUFS/S/PHY/15/14) of Faculty of Science, Cairo University, Egypt.
A wooden pyramid model (Figure 1) of 30 in. height, 45 in. base and 41.5 sides was fabricated locally and used during the present [12].
Unilateral nepherectomy was performed according to Fahmy et al. [12]. Briefly, laparatomy was performed under antiseptic conditions after anesthetization of rats with sodium pentobarbital (50mg/kg body weight; ip). The left kidney was removed following small longitudinal abdominal incision which then closed using a 4-0 silk thread (UNx group). In sham-operated animals, the left kidney was exposed and gently manipulated but left intact (sham group). All animals received normal saline of equal volume at corresponding time points. All the rats received 50μl of 0.2% ropivacaine subcutaneously for the post-operative analgesia.
Fourty two male Wistar rats were assigned equally into three main groups (14 rats for each).The sham-operated control (sham group) and unilateral nephrectomized rats (UNx group). Both groups maintained in their home cages for 24h and 48h. The third group (Pyramid housed group) in which UNx rats housed within the wooden pyramid (6hr/day) for 24h and 48h [13].
At the end of each experimental period, the rats were transferred to individual metabolic cages for 24h and urine was collected. During this time, the rats had free access to water. The collected urine samples were freed from fecal contamination and the urine volume was measured by using the measuring cylinder. The urine samples were centrifuged for 10 minutes and the urine supernatant was then stored at −70 °C until analysis.
Animals were euthanized under deep anaesthesia with sodium pentobarbital. Blood was collected in centrifuge tubes then centrifuged at 3000rpm for 20 minutes. Serum stored at -20 oC until used for biochemical and hormonal assays. Right kidney was removed and immediately blotted using filter paper to remove traces of blood and weighted then suspended in 10% formal saline for fixation preparatory to histological processing.
The appropriate kits (Bio-Diagnostic, Dokki, Giza, Egypt) were used for the determination of sodium, potassium and calcium ions by colorimetric method according to the method described by Trinder [14]; Sunderman and Sunderman [15] and Gindelar and king [16] respectively
Urea was determined by urease-berthelot method using Biodiagnostic kits, according to the method described by Fawcett and Soctt [17]. Total protein was determined by colorimetric method using Biodiagnostic kits, according to the method described by Gornal et al. [18]. Uric acid was determined by enzymatic colorimetric method using Biodiagnostic kits, according to the method described by Barham and Trinder [19]. Creatinine was determined by colorimetric method (End Point) using Biodiagnostic kits, according to the method described by Schirmeister et al. [20]. Creatinine Clearance was determined according to the following equation:
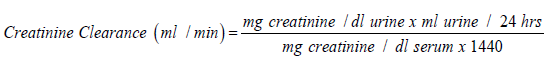
Plasma levels of renin, angiotensin and aldosterone were determined using mouse/rat ELISA kits (Cat# SL0617Ra, SL0061Ra and SL0040Ra, respectively, Sunlong Biotech Co. LTD).
Statistically, the data of the present study were analysed according to Shapiro-Wilk and Kolmogorov analysis. All the statistical analyses done on the bases of the parametric analysis as the raw data were normally distributed. Values were expressed as mean ± SEM. To evaluate differences between the groups studied, one way Analysis of Variance (ANOVA) with the simple t-test was used to compare the group means and P < 0.05 was considered statistically significant. SPSS for Windows (version 15.0) was used for the statistical analysis.
Housing UNx rats either inside their home cages or inside the Egyptian pyramid model induced significant increase (P< 0.05) in the weight of the right kidney as compared to sham group following 24 and 48 h of nephrectomy, respectively (Table 1).
Table 1: Effect of Egyptian pyramid housing on right kidney weight (g) in Unilateral Nephrectomized Rats (UNx).
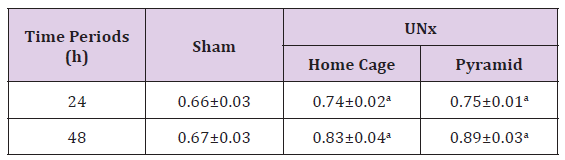
Note: Values are means ± SEM (n=7 per group) a: Significant at (P< 0.05) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
Data recorded in Table 2 showed that unilateral nephrectomy induced significant (P< 0.05) increase in the urine sodium and calcium levels concomitant with marked decrease of their serum levels as compared to the corresponding sham group. However, housing of UNx rats inside the Egyptian pyramid for 24 and 48 h induced significant (P< 0.05) decrease in the urine sodium and calcium levels with increase in their serum level as compared to UNx rats that housed in their home cage. Levels of urine potassium ions decreased significantly (P< 0.05) following nephrectomy for 24 and 48 h as compared to the corresponding sham groups (Table 2). Housing of nephrectomized rats inside the Egyptian pyramid for 24 and 48 h significantly (P< 0.05) increased potassium ions excretion in urine.
Table 2: Effect of Egyptian pyramid housing on some urine and serum electrolytes in Unilateral Nephrectomized Rats (UNx).
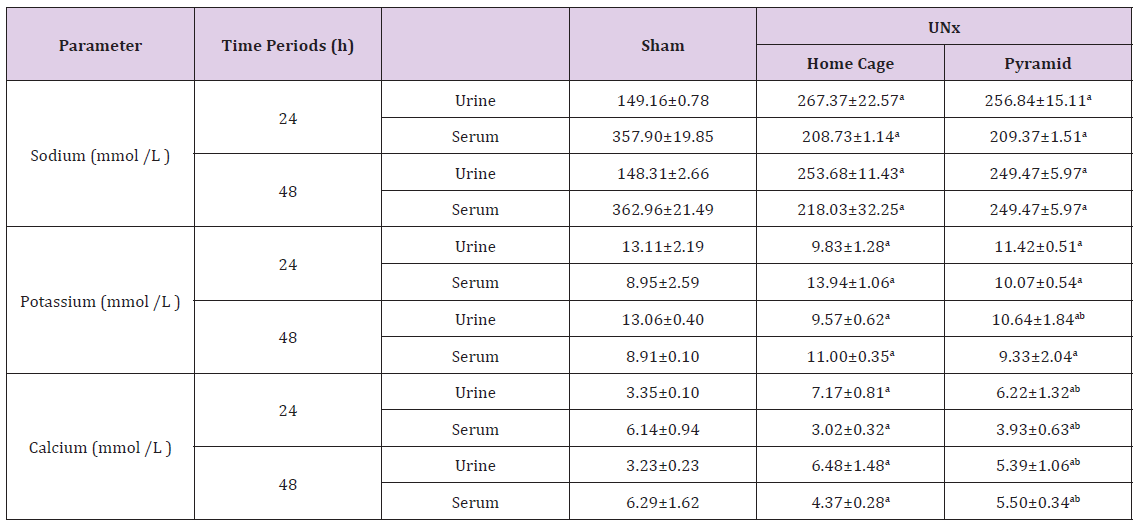
Note: Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
Data illustrated in Table 3 recorded significant reduction (P< 0.05) in the creatinine level of UNx rats either housed inside their home cages or inside the Egyptian pyramid model for 24 and 48 h as compared to the corresponding sham groups. However, serum creatinine level significantly increased (P< 0.05) in UNx rats either housed inside their home cages or inside the Egyptian pyramid model for 24 and 48 h as compared to the corresponding sham groups (Table 3). The creatinine clearance significantly decreased (P< 0.05) in UNx rats housed in their home cages and inside the Egyptian pyramid model following 24 h of nephrectomy. On the other hand, creatinine clearance increased significantly (P< 0.05) only after housing UNx rats inside the Egyptian pyramid model following 48 h (Table 3).
Table 3: Effect of Egyptian pyramid housing on serum creatinine and creatinine clearance levels in unilateral nephrectomized rats (UNx).
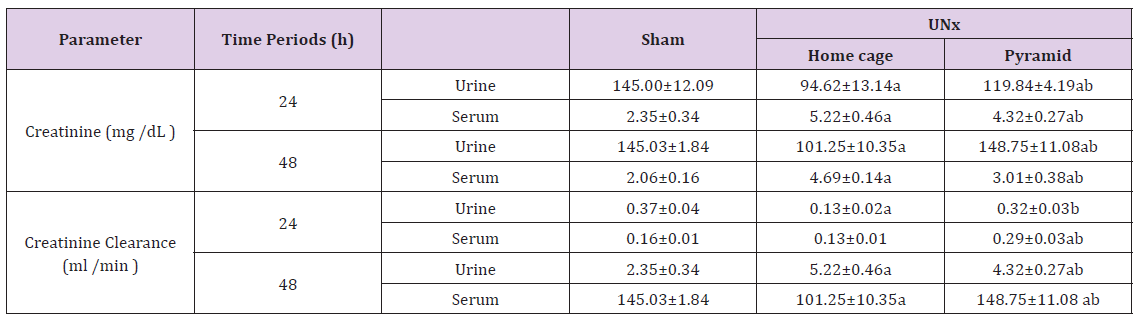
Note: Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
Figures 2 & 3 demonstrated significant increase (P< 0.05) in proteinuria with concomitant significant decrease (P< 0.05) in total serum protein level in home cage UNx group as compared to sham group after 24 and 48 h following nephrectomy. The obtained data also showed significant decrease (P< 0.05) in the levels of urine urea and uric acid in home cage UNx rats as compared to sham group (Figures 4-7). On the other hand, their levels increased significantly in the serum (P< 0.05) of home cage UNx rats after 24 and 48 h following nephrectomy. However, housing of rats inside the Egyptian pyramids for 24 and 48 h significantly (P< 0.05) ameliorate these effects and restore their levels towards the control values (Figures 4-7).
Figure 2: Effect of Egyptian pyramid housing on urine protein level (g/dl) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
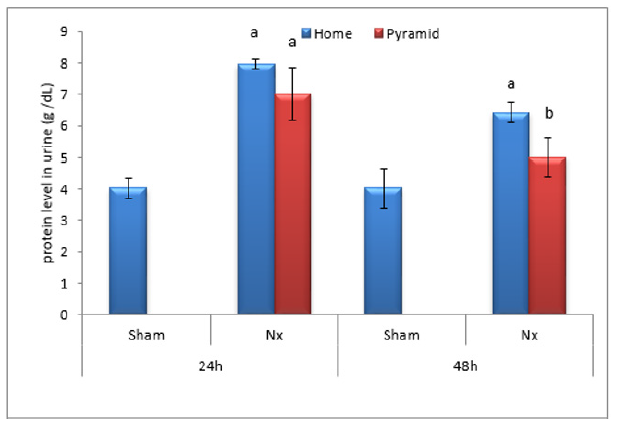
Figure 3:Effect of Egyptian pyramid housing on serum protein level (g/dl) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
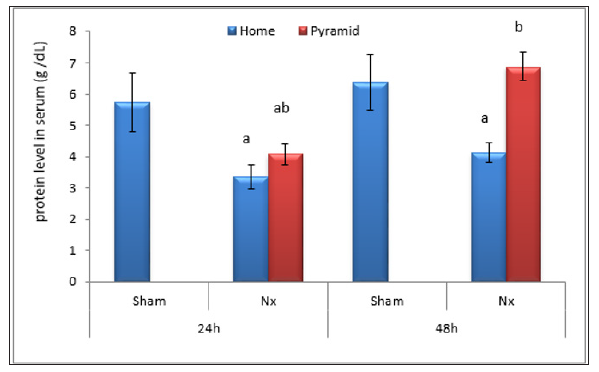
Figure 4: Effect of Egyptian pyramid housing on urea level in urine (g/dl) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
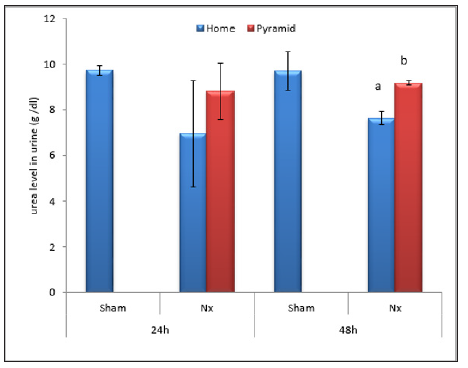
Figure 5: Effect of Egyptian pyramid housing on serum urea level (g/dl) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.

Figure 6: Effect of Egyptian pyramid housing on urine uric acid level (mg/dl) in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
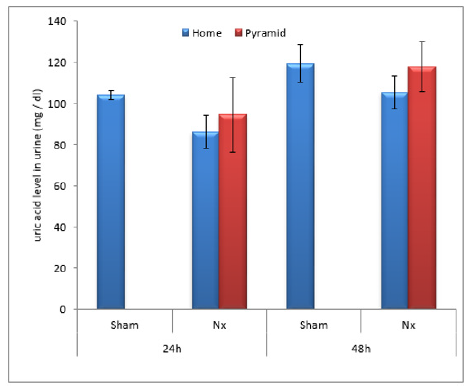
Figure 7: Effect of Egyptian pyramid housing on serum uric acid level (mg/dl) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
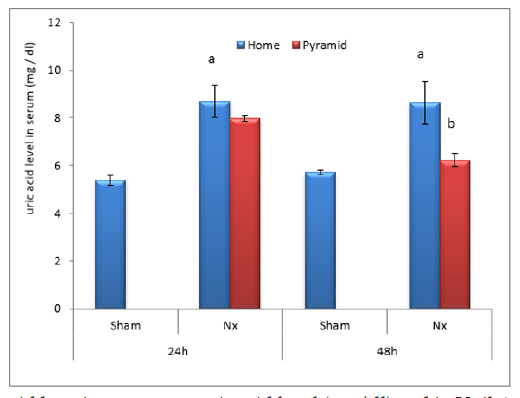
Figure 8: Effect of Egyptian pyramid housing on renin level (mmol/l) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
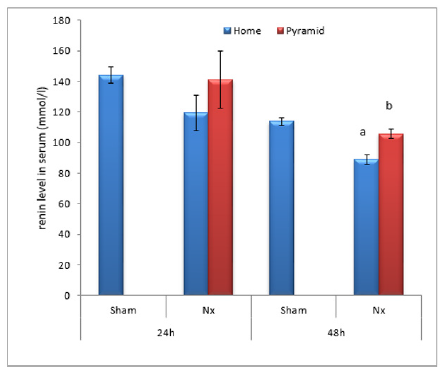
Data illustrated in Figures 8-10 showed significant decrease (P< 0.05) in the levels of serum reinin, angiotensin and aldosterone following unilateral nephrectomy in rats as compared to sham group. Levels of the studied hormones significantly increased after housing UNx rats inside the Egyptian pyramid for 24 and 48 h.
Figure 9: Effect of Egyptian pyramid housing on angiotensin level (mmol/l) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
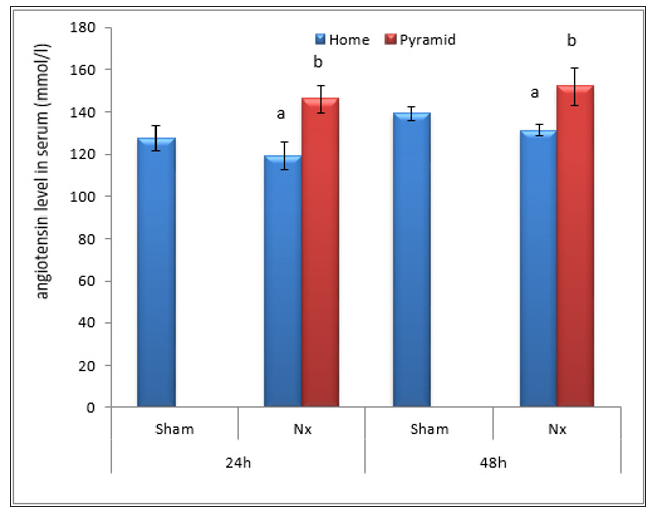
Figure 10: Effect of Egyptian pyramid housing on aldosterone level (mmol/l) and in Unilateral Nephrectomized Rats (UNx). Values are means ± SEM (n=7 per group). a: Significant at (P< 0.05 ) as compared to the corresponding sham group. b: Significant at (P< 0.05 ) as compared to the corresponding home cage group.
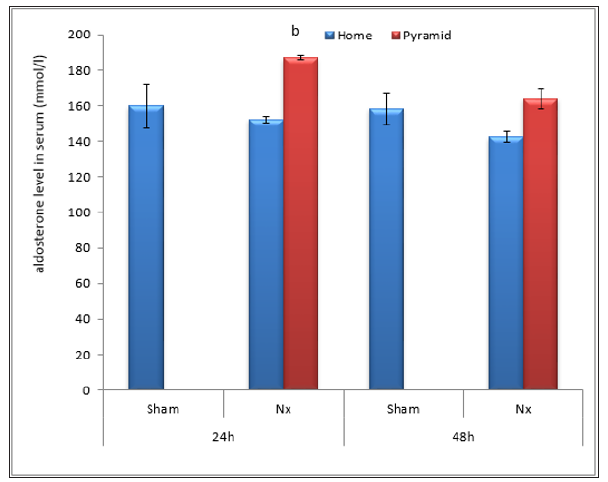
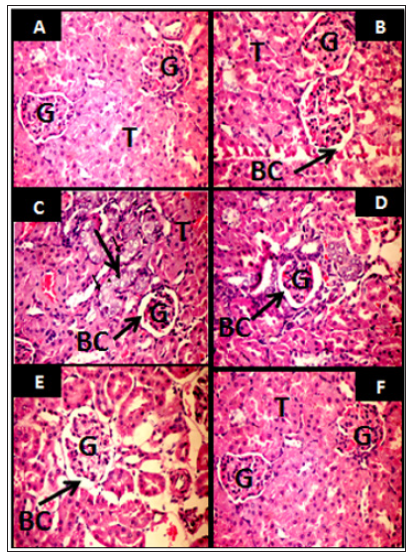
Figure 11 illustrate the histological alterations in kidney of UNx rats following housing inside the Egyptian pyramid model. Figure 11A & 11B represent microscopic examination of kidney in sham groups showing normal renal tubules and renal glomeruli. Dilated glomeruli with focal area of vacuolated renal tubules (arrows) were observed in UNx rats housed inside their cages for 24 and 48 h (Figure 11C & 11D). Housing UNx rats for 24 and 48 h inside the pyramid model demonstrated healthy renal tissue (Figure 11E & 11F).
Figure 11: Light micrographs of representative kidney sections (Haematoxylin and Eosin stain, x 400) from sham rats (A,B), Unilateral Nephrectomized (UNx) rats housed inside their home cages for 24 and 48 h respectively (C&D) and UNx rats housed under the pyramid model environment for 24 and 48 h respectively (E&F). G: glomerulus, BC: Bowman’s capsule, T: tubules.
The pyramid shape is being seen as a supernatural source of energy of the universe. The energy power inside the pyramids is a way to recover the Electro-Magnetic Fields (EMF) that have been essential for the communication of living cells [21]. Now the pyramids are being used very efficiently for balancing the centres of energy in human body, medical healing and anti-stress effects [22,23]. To our knowledge the effectiveness of the Egyptian pyramid model housing in preventing the renal failure following surgical resection of renal carcinoma not studied till now. Thereby, the present study postulated that housing of nephrectomized rats in the early stages (24 and 48h) inside a model of the Egyptian pyramid may be preventing renal failure that concomitant with the Unilateral Nephrectomy (UNx).
One of the best recognized mechanisms of renal failure following nephrectomy is hyperfiltration in the remaining nephrons, which is then thought to induce focal and segmental glomerulosclerosis, further damaging the remaining nephrons [24,25]. Thereby, the remaining kidney increases in size in response to hyperfiltration and begins to compensate for its missing partner [26,27]. The loss of renal mass or UNx is associated with functional and morphological adaptations in the remaining tissue. The most prominent response after UNx is an increase in size and weight of the remaining kidney. In consonance with the previous suggestion, the present study confirmed the finding of Gava et al. [28] and Mjøen et al. [29], who recorded significant increase in the weight of right kidney following UNx in rats. Moreover, Abdellatif et al. [30] reported that UNx may be associated with enlargement and hypertrophy of the remaining kidney.
However, the compensatory renal growth occurred in UNx rats either housed in their home cages or inside the pyramids. To determine if the compensatory renal growth in UNx rats accompanied with renal failure or not, the present study continued to investigate kidney function. The serum levels of urea and creatinine are often considered as reliable markers for measurement of renal function [31]. In agreement with the report of Baracho et al. [32] and Bai et al. [33] the present study recorded marked decrease in urea, uric acid and creatinine levels in the urine of UNx rats housed inside their home cages concomitant with marked increase in their levels in the serum. The impairment in the levels of urea and creatinine in the urine and serum following nephrectomy may indicates destructive in the ability of the kidneys to filter these waste products from the blood and excrete them in urine [34,28]. Housing UNx rats in the pyramid model for 24 and 48 h induced enhancement in the kidney function as indicated by improvement in the levels of studied parameters.
Proteinuria is thought to reflect glomerular hypertension and hyperfiltration in the single kidney and to anticipate renal functional decline [35]. In consonance with the finding of Ibrahim et al. [36] and Zhong et al. [27], the present investigation recorded significant proteinuria in UNx rats housed in their home cages for 24 and 48 h. Proteinuria is considered to be a marker of dysfunction of the glomerular filtration barrier [37]. The obtained results demonstrated that housing UNx rats under the pyramid model environment can significantly reduce proteinuria in the early stages. Renal function is most widely assessed by Glomerular Filtration Rate (GFR), either measured or estimated. Direct measurements of GFR are uncommonly performed on partial nephrectomy patients. Therefore, most partial nephrectomy renal function studies rely on estimates of glomerular filtration based on serum creatinine [38]. In conjunction with the report of Zorn et al. [39], Chapman et al. [40]; Nutter et al. [41] and Saito et al. [42], UNx is associated with an acute and significant drop in the GFR which may be due to the immediate loss of renal mass. The data recorded in the present investigation showed more increase in the GFR of the UNx rats housed inside the pyramid model for 24 and 48h which may suggest predominantly hemodynamic response and more efficient hyperfiltration [35,26]. In agreement with the reports of Wesson [43] and Bohlouli et al. [44], the increment in the GFR could be due to compensatory changes in the remaining kidney. However, the increase in the GFR following housing UNx rats under the pyramid environment may be due to an increase in kidney weight and an increase in the GFR per gram of kidney [45]. In addition, the GFR of the remaining kidney need to be increased to maintain fluid and electrolyte balance, through adjustments in vasomotor control of the microvasculature [46].
The concentration of Na+ and K+ electrolytes in the extracellular fluid is maintained by the kidneys [47]. The present study supported the reports of Gava et al. [28]; Calunga et al. [48] who recorded significant rise in the rate of sodium excretion in UNx rat’s concomitant with its increase in the serum. This increase may be attributed to a decline in the fractional sodium reabsorption in proximal tubules [49]. The high rate of sodium, potassium and clacium excretion in urine may be due to the increased filtration burden on the remnant glomeruli which could leads to increased excretion of ions from and the decreased accumulation of ions in the plasma [50]. Housing of Nx rats inside the Egyptian pyramids did not affect levels of electrolytes in urine and serum which may suggest that housing inside the pyramide model ameliorate kidney function through certain mechanism other that homeostasis of electrolytes.
The Renin‐Angiotensin System (RAS) is a major regulator of sodium and water homeostasis, vascular resistance, and ultimately arterial blood pressure [51]. The classical RAS consists of a circulating endocrine system in which the principal effectors’ hormone is angiotensin II. Angiotensin II is a key biologic peptide in the Renin-Angiotensin System (RAS) that regulates blood pressure and renal hemodynamics [52]. The present study supported the finding of Dilauro et al. [53], who reported reduction in plasma Ang II following nephrectomy in rats which may be due to secondary suppression of systemic RAS. Normally, an increase in fluid delivery to the macula densa, will initiate afferent arteriolar vasoconstriction due to activation of the Tubuloglomerular Feedback (TGF) system [54]. The TGF feedback is a negative-feedback system operating within the juxtaglomerular apparatus that can regulate GFR by changing arteriolar resistance and hence blood flow and pressure into the glomerular capillaries [55]. Housing UNx rats inside pyramid model restore normal level of RAS which may indicate that the pyramid provide good environment for adaptations after nephrectomy without negative consequences for renal function, despite the fact that glomerular hypertension and hyperfiltration can cause glomerular damage. Moreover, the energy field developed within the pyramid may increase the GFR by increase in the renal blood flow which stimulate renin production and enhance RAS system that increasing sodium reuptake.
The present study showed that housing nephrectomized rats inside model of the Egyptian pyramid may be a potential new strategy prevents CKD following renal tumor resection. Also, the current study suggested that pyramid environment plays an important role in ameliorating the serious effects of nephrectomy by enhancing kidney function. However, further studies are necessary to elucidate the mechanism by which pyramid environment reversed the actions of nephrectomy in rats.


