Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Abiya SE, Odiyi BO, Ologundudu FA*, Akinnifesi OJ and Akadiri S
Received: December 10, 2019; Published: January 02, 2019
*Corresponding author: Ologundudu FA, Department of Biology, Nigeria
DOI: 10.26717/BJSTR.2019.12.002276
The unwanted release of environmental contaminants predisposed by mining activities had reached an alarming proportion that deserves attention. Hence, the purpose of this study was to determine the degree of heavy metal contamination which soil and plants were exposed to in Ijana gold mining site, southwestern Nigeria. To this, Zinc, Arsenic, Cadmium, Lead, Nickel, Chromium, and Copper concentrations were measured using Atomic Absorption Spectrometry. Obtained values were used to evaluate the degree of soil pollution and plant contamination using physicochemical analysis, bioaccumulation factor and translocation factor of metals into plant in surrounding mine site. Zinc and Lead show a slightly higher presence than other metals tested. Mean concentrations of Zn (0.70 mg/kg), As (0.09 mg/kg), Cd (0.13mg/kg), Pb (0.216kg/mg), Ni (0.08mg/kg), Cr (0.148mg/kg), Cu (0.629mg/kg) in soils around the mining area were considerably the same with the concentration of metal accumulated in plant respectively. All metal tested showed minimal accumulation in plants. Translocation factor also implicated Zn to be the highest among all the heavy metals analysed.
Keywords: Bioaccumulation; Translocation; Pollution; Tailings
Amongst many anthropogenic activities, mining has been identified with the potential of impacting negatively on the quality of the environment [1,2]. Mining causes the destruction of natural ecosystems by altering soil, vegetative covers and covering of organisms beneath excavation sites Cook and Johnson. Aside the physical habitat destruction with accompanying the loss of biodiversity resources, the accumulation of pollutants in different media have been recorded around mining sites Getaneh [3]. Therefore, mining sites portend great toxicological challenges for the surrounding ecosystems and on human health Franco-Hernández [4]. Like any exploitative activity, the excavation of mineral resources produces negative impacts upon the hydrospheric, atmospheric and lithospheric components of the environment Eisler and Franco et al. [4-6]. In gold mining, like many metallurgical extractions, crystallographic bonds are broken in the ore mineral in order to recover the desired element or compound Lottermoser [7].
During gold mining, large quantities of waste are produced. Over 99% of extracted ore in gold mining are released into the surrounding environment as waste Adler et al. [8]. One of the wastes that have been implicated around mining sites is heavy metals. Heavy metals have received global attention of researchers, owing to their deleterious effects on plants, especially those on vegetative and generative plant parts. Ekaterina & Jeliazkova [9]. Due to variations in the physical and chemical properties of soil, heavy metals in tailings can be translocated and accumulated in plants and animals. Even in low concentration, heavy metals can persist in soil from and can enter into food chain through plant uptake Donkor [1]; Gulam et al. Some heavy metals like Lead, Arsenic, Mercury, Canadium, are not essential for plants growth, since they are not known to perform any physiological function in plants. Other metals like, Iron, Copper, Nickel Manganese, Cobalt, Molybdenum, and Zinc are essential elements required for normal plant growth and metabolism, but when they are above desired concentrations, could constitute poisoning to individual plants Garrido et al. [10,11].
Among all the heavy metals, cadmium (Cd) is a highly toxic metal for both the plants and animals as well as for human beings. Cadmium enters into soil-plant environment mainly through anthropogenic activities. Elevated Pb in soils may decrease soil productivity and if in very low concentrations, Pb may also inhibit some important plant processes i.e. photosynthesis, mitosis, water absorption and vegetative growth Bhattacharyya et al. [12]. Studies have also shown the genotoxic effect of chromium on cells. Cr (VI) has been found to 100 times more toxic and 1000 times more mutagenic and carcinogenic compared to Cr (III) Costa [13]. Heavy metals also exert toxic effects on soil microorganism hence results in the change of the diversity, population size and overall activity of the soil microbial communities Ashraf & Ali [14]. In order to evaluate the damages that gold mining activities exert on the environment, especially in areas in which crude methods of mining is still largely used, there lies the need to assess the extents of pollution. These must be based on studies about waste properties, heavy metals content and their relation to soil and plant. Hence, the objective of this study is to
a) Determine the physicochemical properties of soil from Ijana goldmine.
b) Determine the concentration of heavy metals found in soils and plants at the goldmine and
c) Compare the result of the physicochemical properties of the soil and heavy metal concentration in plants and soils at Ijana goldmine with that of a control site.
The study was conducted in a gold mining site in Ilesha, Osun state. The site is located at Ijana of Atakunmosa west local government in Osun state. Mining site is on Latitude 7.573° N and longitude 4.678°E and the control site on Latitude 7.578° N and longitude 4.679°E.
Soil and Plant samples were randomly collected at Ijana goldmine site. Control samples were taken at a site a few kilometers away from the mine site. The control sites have no record of mining activities and also have limited human interference. Three soil samples were collected at the surface (0-15cm) and subsurface (15-30cm) respectively using a hand trowel and meter rule at both mine and control sites. Plant samples (Chromolena odorata) which was common to both the mine and control sites were carefully uprooted ensuring that the roots remained intact. Both the soil and plant samples were correctly labeled and bagged before taking to the laboratory for analysis. Each soil sample was air-dried for 7 days and sieved to <2 mm prior to analysis. for physico-chemical properties including pH, potassium (K), calcium (Ca), magnesium (Mg), sodium (Na), CaCO3, organic matter, total organic, heavy metals. Total K, Ca, Na and Mg concentrations were determined using flame emission after digestion of the composite samples with boiling 2 M HNO3 for 2 h. Porosity and Bulk density, Organic matter contents and other soil and plant analyses were tested using standard methods.
The pH and conductivity of soil and plant material were carried out using standard methods as described by Tahar & Keltoum [15]. The porosity and bulk density of soil samples in mining site and control site were tested using the methods of Danielson & Sutherland [16]. Calcium and magnesium (Exchangeable bases) content of soil were assayed following standard methods. The organic composition of the soil was tested using the Walkley-Black Wet oxidation method Walkley & Black [17]. The cation exchange capacity was determined using the ammonium saturation method described by Jackson. Bioaccumulation factor of metal concentrations in the receiving plant shoot and were evaluated with concentration in the soil under standard methods of Radulescu et al. [18] and the formula is shown below.
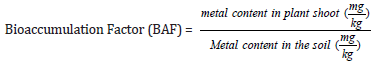
Also, the concentrations of metal translocated from root to shoot of the plant around tested site were determined according to the method of Abdul et al. and the formula is shown below
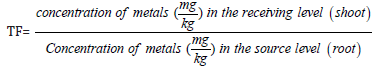
The metal analyses of samples (Ni, Cd, As, Cu, Zn, Pb and Cr) were carried out by using an Atomic Absorption Spectrophotometer (AAS).
Soil Physico-Chemical Properties
For the soil physico-chemical properties, the pH of both the mining and control sites for surface (0-15cm deep) and subsurface (15-30cm deep) level are approximately neutral and within minimum acceptable limits. However, the pH of the mine site soil at 0-15cm was found to be slightly acidic (5.91) than the control (6.85). Also, soil sample at the subsurface (15-30cm) level were generally less acidic (Table 1). Effects of such decrease in soil pH is reported to result in an increase in heavy metal absorption by plants due to dissolution of metal carbonate complexes releasing metals into solution during the rainy season Mapanda et al. [19]. In the mine soils, soil conductivity was found to be higher than the control soils both at the surface and subsurface levels. At 0-15cm (surface layer), the mining site gives a mean value of 574 as against the control site 142. At the sub-surface layer, the mining site gives a mean value of 1180.33 against the control site 135.33 (Table 1). Conductivity at the control site falls within WHO [20] permissible limit of 16-175, that of mine site far exceeds this limit.
Conductivity at the mine site was found to be significantly different when compared to that of the control at the surface level (0-15cm). This is probably due to the release of ions which ordinarily will be bound to rocks but are broken down and washed off during the gold mining process. These results conform to the findings by Bjuhr [21] in his studies of mine soils. The mean value for the bulk density of the soil surface level of the mining site was found to be 1.41 and control 1.21 while the bulk density of the soil sub-surface level of the mining site was found to be 1.32 and control 1.21. With respect to physicochemical analyses carried, only bulk density and calcium has significant difference across the two surface levels.
The highest levels of Zn, Cd, Pb, and Cu were found in the soil from mine site (Table 2). The levels of Zn were lower than the range expected in contaminated soils i.e. 20-300 mg/kg. The levels of Ni found in the soil samples were also below the normal range of 1-110 mg/kg reported for uncontaminated soils. For both surface and subsurface soil in the mine and control sites, arsenic was found to be the element with the least concentration. The concentrations of all metal recorded in the two surfaces are not too different from one another. Soil samples collected top surface (0- 15cm deep) had slightly lower levels of Pb than the normal than the sub-surface (15-30cm deep). The copper, chromium and arsenic concentrations were, however, lower than the values reported for typical uncontaminated soil. This result conforms to the findings of Tahar & Keltoum [15]. A high contamination of the soil with metal could elicit deleterious effects on microbial activities, Dai et al. [22] provoking a low organic matter mineralization needed for plant growth. The apparent increase of heavy metals concentration in mine site compared to the control site almost certainly confirms the mining waste as the potential source of soil contamination and their accumulation in plants. An increasing level of these metals presents the site as potentially hazardous and could alter food chain and biological life in the environment.
In the present study, the results obtained showed that heavy metal (Zn, As, Cd, Pb, Ni, Cr and Cu) concentrations varied in the plants parts. In both the root and shoot, the concentrations of heavy metals were found to be higher in the mine site than the control site (Table 3). Of all the level of heavy metal concentrations, Zinc and Lead shows to be significantly higher in the mine site when compared to the control. Zinc as a natural soil element play essential functional and structural role in plant growth. It usually occurs in low concentrations and does not pose a toxicity problem for plants, but at higher concentration could pose some risk on plants Ahmad & Erum [23]; Paschke et al. [24]. In Table 3, the values of zinc in both root (1.205mg/kg) and shoot (1.017mg/kg), though higher in mine site than the control is much lower compared to environmental quality standard range of 100-400 mg/kg.
Note: *- Significant
Reports from various studies have implicated lead accumulation in vegetative plant part declining with distance from possible contamination sites Little [25]. This is also noticed in the present study. The rate of deposition of lead on vegetative cover is about four times greater than on bare soil. Little & Wiffenn [26]. In the present study the lead concentration recorded in the two plant parts was quite low compared environmental quality standard range of 50 mg/kg. Arsenic tested in plants is below the toxicity threshold for above ground tissues of 3-10 mg/kg. Nickel play vital metabolic function in higher plants, the value of nickel in study root and shoot: was 0.079 and 0.088 mg/kg, respectively. These values of nickel were quite lower than environmentally acceptable standard of 1-5 mg/kg. Ahmad & Erum [23]. Naturally without pollution, the copper concentration in soil is 20 ppm. In this study, Copper concentrations in plants species were not higher than the toxic values. The little concentration recorded might be due to the presence of copper in minerals which can be released only by very slow disintegration processes Radulescu et al. [18].
Cadmium is a non-essential nutrient, the value of Cd is recorded in both plant and shoot is equally low. Compared with the other metals cadmium is found to leach more in soil, with increased availability in plant Ahmad & Erum [23]. It has also been found that cadmium pollution without co-contamination by zinc is rare. Clemente [27]. Although all metals assayed were still within the permissible limit, the presence of metal tends to be higher in the root part than in the shoot. And also higher in the mine site than in the control.
Translocation factor was calculated as the ratio of heavy-metal concentrations in plant shoot to those in the corresponding root. According to the previous research results Baker & Brooks [28]; Abdul et al, TF value should be below 1 (TF >1). This study shows the results of Translocation Factor (TF) of heavy metals from shoot to root. From the result in Table 4, Zn was found to have the highest translocation factor in C. odorata at the mine site, followed by Cu. For both metals, the translocation factor is less than 1. (0.96 at the mine site against 0.72 at the control site and 0.95 for Cu at the mine site against 0.85 at the control site). This implies that the shoot of this plant hold these metals than others. Therefore, the order of uptake capability from shoot to root is Zn > Cu > Cd > Pb >As > Ni.
Bioaccumulation factor (BAF), calculated as the ratio of heavymetal concentrations in plant shoot to those in the corresponding soil. According to Gautam et al. [29] BAF value should be below 1. In this study only, Zinc had a BAF>1, other metals are generally less than 1. This result indicates that the concentration of Zn in the plant shoot is higher than that in soil. Zinc has been confirmed to be easily absorbed by plants Mijovilovich et al. [30]. At the control site, none of the heavy metals had a bioaccumulation factor of more than 1. Copper was the highest at 0.67. Zn, As, Cd, Pb, Ni, Cr and Cu had bioaccumulation factor 0.01, 0.01, 0.17, 0.06, 0.14 and 0.67 respectively (Table 5).
The concentration levels of heavy of metals (Zn, As, Cd, Pb, Ni, Cr, and Cu) in the soil and plants samples from Ijana mining site were generally low and found to be within the World Health Organization (WHO) permissible levels. This could probably be due to the fact that most mining operations in the site are low scale and artisanal in operation unlike other sites where mechanized mining techniques could predispose release of more pollutants and tailings. Therefore, the soils at Ijana at the time of this study doesn’t present significant contaminations, thus the soil environment around the mining field are yet to be impacted negatively by the mining activity. Increased mine expansion would also necessitate continued assessment for possible pollution around the mine site.


