Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
CPanduranga Murthy G1*, Leelaja BC2, Ravishankar HG3, Dharshan Raj CG4, and Rajesh Kumar5
Received: November 19, 2018; Published: December 10, 2018
*Corresponding author: Panduranga Murthy G, Maharaja Institute of Technology Thandavapura, India
DOI: 10.26717/BJSTR.2018.11.002171
Sida glutinosa Comm. ex Cav.- an aboriginal ethno-medicinal plant drug belongs to family Malvaceae, commonly called as ‘Sticky Fanpetals’. It is a sub-shrub which is available in the forest at shady areas along Ravines. The plant was collected at Biligirirangana Hills (B.R. Hills) of Chamarajanagar district, Karnataka, India and taxonomically identified. The therapeutic information was recorded from traditional medicine men during interaction with tribal healers at B.R. Hills. In the study, methanol extract of Sida glutinosa (G) was preliminarily screened for Physico-chemical and Phytochemical properties. Further, the antioxidant activity both in vitro and in vivo was carried-out. The neuroprotective study was explicitly carried out using a model system, Drosophila melanogaster (Oeragon K) strain adult male flies. The result reveals that, the extract G exhibited concentration dependent DPPH scavenging activity. The oxidative stress markers employed to access in vivo antioxidant property of G included lipid peroxidation products malondialdehyde (MDA) and hydroperoxide (HP), reduced glutathione (GSH). The modulatory effect of G on superoxide dismutase (SOD) and catalase (CAT) was also determined. The oxidative stress was induced by using paraquat at 15 mm. The concentration of extract for studies was fixed based on LC50 values. There was a significant annihilation in the levels of MDA and HP in case co exposure of G with Par treated flies’ homogenate. Finally, the level of SOD and CAT was brought to near basal level in the homogenate of flies co exposed with G and Par. In negative geotaxis assay; it was found that, G was able to rescue the flies significantly from deteriorating locomotors dysfunctions. The extract G also exhibited significant antibacterial property against the tested strains.
Abbrevations: MDA: Malondialdehyde; HP: Hydroperoxide; GSH: Glutathione; SOD: Superoxide dismutase; CAT: Catalase; ROS : Reactive Oxygen Species; RNS: Reactive Nitrogen Species; PD: Parkinson’s Disease; SCZ: Schizophrenia; AD: Alzheimer’s Disease; NADPH: Nicotinamide Adenine Dinucleotide Phosphate; DPPH: 1,1-diphenyl-2-picryl-hydrazyl; TBA: thiobarbituric acid; SDS: Sodium lauryl sulphate; OPT: o-phthalaldehyde; TEMED : N,N,N,N Tetramethyl ethylenediamine; EDTA: Ethylenediamine tetra acetic acid; ANOVA: One-Way Analysis Of Variance; MIC: Minimum Inhibitory Concentration
Aerobic organisms produce several reactive free radicals continuously in cells during respiration, metabolism and phagocytosis. The free radicals formed within the cells can induce multiple chemical changes in cellular components like membrane lipids, DNA and proteins, which can eventually lead to cell death. The collective terms “Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)” refer to a variety of free radicals such as superoxide, hydroxyl, peroxyl, nitric oxide, nitrogen dioxide radicals as well as for nonradical reactive intermediates like hydrogen peroxide (H2O2) and peroxynitrite (ONOO.) etc. Their excessive production, termed as “Oxidative Stress” has been implicated in many pathological disorders like heart disease, cancer and ageing [1]. Oxidative stress is a harmful condition that occurs when there is an excess of free radicals or a decrease in antioxidant levels. The evidence to date for oxidative stress in Parkinson’s disease (PD), Schizophrenia (SCZ), Alzheimer’s disease (AD) and other neurodegenerative diseases is strongly persuasive. The clinical studies showed that several events associated with Alzheimer’s are capable of stimulating production of free radicals and depletion of antioxidant levels. As pointed out, whether oxidative stress is eventually proven to be primary or secondary in etiologic progression, the therapeutic rewards of antioxidants are likely to be substantial. Clearly, strategies are aimed at limiting free radical induced oxidative stress and damage may slow the progression of neurodegenerative diseases [2].
Paraquat (1,1-dimethyl-4,4-bipyridynium dichloride) is a quaternary nitrogen herbicide and highly toxic substance for humans and animals; many cases of acute poisoning and death have been reported [3]. The toxicity of paraquat is due to the generation of the superoxide anion which can lead to the synthesis of more toxic Reactive Oxygen Species (ROS) such as hydroxyl radicals and hydrogen peroxide [4]. On the other hand, the oxidation of reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) because of paraquat administration results in the disruption of biochemical processes requiring NADPH [5]. The use of antioxidant compounds provides an easy and convenient way of testing the validity of the free radical theory using Drosophila melanogaster as model organism. The organisms are easier to culture and manipulate under laboratory conditions than the mammals. The administration of the test compounds can be done easily by adding the dissolved compounds to the food medium thus ensuring their uptake by the flies [6].
Sida glutinosa is a sub-shrub plant which is available in the forest at shady areas along ravines & native of tropical America and naturalized in Asia. It belongs to the family Malvaceae, commonly called as ‘Sticky Fanpetals’ [7]. The roots and stems of the plant contain ephedrine, an important alkaloid; besides this, traces of sitosterol and palmic, stearic etc. have also been isolated from this plant. It was reported that the main alkaloid present in Sida is asparagin. Besides this fatty oil, phytosterol, mucin, potassium nitrate, resins and acids are also known to be found in the plant extract. It is also reported that Sida does not contain any tannin or glycoside. The recent studies reported that, ephedrine and yephedrine are the major alkaloids found in aerial parts of the plant. Besides these two, some other chemical compounds were isolated from the aerial parts of Sida are 6-phenyl ethyl amine, carboxylated tryptomines, qunazoline, hypaphorine, vasicinol etc. Different species of this plant have been reported to contain cryptolepine also [8]. It was also reported that the extract of Sida was useful in the treatment of fevers, ophthalmia, rheumatism, leucorrhoea, micturition, gonorrhea, colic, neurological disorders, analgesic, anti-inflammatory and general impediment. The root extract of Sida plant was also exhibited wound healing property [9].
The plant extracts of Sida glutinosa is being practiced in tribal medicine formulations, where it is used to cure diarrhea, stimulating and nutritive followed by wound related issues. The rejuvenating action of this herb extends to the nervous, circulatory and urinary systems. It was reported that it has diuretic effect and is useful in the treatment of urinary problems, including cystitis. It is also used as anti-inflammatory and in treatment of infections and bleeding disorders. By considering all these medicinal properties reported by the researchers and usage of the plant in the tribal drug formulations, we worked towards scientific validation of the drug through a preliminary appraisal of physicochemical, Phyto-chemical properties, antimicrobial, in vitro, in vivo antioxidant activity and neuroprotection efficacy of leaf extract of Sida glutinosa was carried out for the first time.
A less known ethno-medicinal plant Sida glutinosa was collected at Biligirirangana Hills, Karnataka, India. The plant material was identified taxonomically and the validation was made by Prof. G. P. Murthy, Scientist and Head, CSRF, Tumkur, India (BRT/024/2008). The information on therapeutic uses was recorded during interaction with tribal (Soliga) medicine men. The plant leaves were collected and used for further studies (Figure 1).
Preparation of the Methanol Extracts: The leaves were collected and rinsed with distilled water. They were dried for 5 days in the dark at room temperature. After five days, they were ovendried for 1 hour at 60°C. They were made into fine powder and the crude extract was prepared in the methanol. The extract was filtered through a Whatman, No. 1 filter paper. The extract was evaporated under reduced pressure using rotary evaporator. The powder was stored in dark glass bottles for further use.
Physico-chemical Characterization: The powder of leaves of Sida glutinosa were subjected to physicochemical study for determination of foreign organic matter, moisture content, Total ash value, Acid insoluble ash, Water soluble ash, using the methods described by Indian Herbal Pharmacopeia and United State Pharmacopeia [10].
Preliminary Phyto-chemical Analysis: Phyto-chemical screening of the Methanol extract of Sida glutinosa was carried out to identify the major constituents in the sample. The standard procedure was used to determine Phyto-chemical properties [11,12].
Antimicrobial Studies: The extract G was screened for their antifungal activity against Aspergillus niger, Candida albicans. The antibacterial property was evaluated against Staphylococcus aureus, Salmonella typhi, and Bacillus subtilis strains by disc diffusion method. The compounds were dissolved in DMSO and antimicrobial activity was determined by serial plate dilution method [13].
DPPH Radical Scavenging Activity: The DPPH assay was carried out according to the standard method with some modifications. The stock solution was prepared by dissolving 24 mg DPPH with 100mL methanol and then stored at -20°C until needed. The working solution was obtained by mixing 10mL stock solution with 45mL methanol to obtain an absorbance of 1.17, 0.02 units at 517 nm using the spectrophotometer. The solution of compounds at 5μg/ml concentration was prepared in ethanol. The different aliquot of stock solution was used for estimation. It was diluted to 4 ml using distilled water. To this 1ml of 1,1-diphenyl- 2- picryl-hydrazyl (DPPH) solution in methanol was added. The mixed solution was incubated at room temperature for 30 min. The absorbance of stable DPPH• was read at 517 nm using UV-vis. spectrophotometer and the remaining DPPH was calculated [14].
Preparation of Wheat Cream Agar Medium and Culturing of Flies: D. melanogaster (Oregon K) adult males (8-10 days old) were obtained from Drosophila stock center, Department of Studies in Zoology, University of Mysore, Manasagangotri, Mysore, Karnataka, India. The flies were maintained at 22 ± 1°C and 70-80% relative humidity, fed on a standard wheat cream agar medium seeded with yeast. The medium was prepared according to standard protocol of the media (100 mL) containing 10g wheat flour, 10g jaggery, 1g agar and 0.75ml propionic acid (Antifungal Agent) few granules of yeast were also added. After 24 hours flies were transferred to fresh media bottles to avoid sticking of flies to media. Whenever required, the flies were exposed to the fumes of diethyl ether in a small airtight glass container for 1 min. for observation under stereo zoom microscope and for other studies.
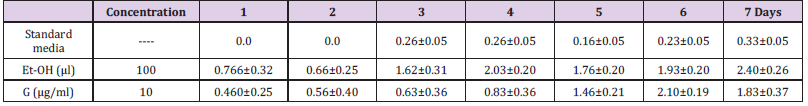
Preparation of extract for feeding the Flies: The plant extract (G) was dissolved in ethanol. The G was then introduced into the medium at semisolid state and mixed well and could solidify. Adult males (50 no.) were introduced into the vials containing media. The safety evaluation of G was dissolved in Et-OH. The toxicity of Et-OH was checked in the medium by rearing the flies in media with and without 100μL Et-OH. Since there was no mortality in the flies reared on medium containing 100μL Et-OH, for all experimental purposes wheat cream agar medium was used with 0.5% DMSO. Further studies were carried out to find out whether the compounds are causing mortality in the experimental batches. In this set of experiments, the males (test) were fed on a medium containing G 10, and 20 μg/mL concentrations. In each vial 4 mL of control food or food containing the compounds were added. The vials were closed with cotton stoppers. Lethality due to compounds was monitored by counting dead flies every 24h up to 7 days, and data was expressed in terms of percentage mortality [15].
Whole Body Homogenate Preparation: Sodium-phosphate buffer 0.1M, (pH 7.4) was used for preparing whole body homogenates. Males (30 no.) from control and tested groups were used for this purpose. After homogenizing, the samples were centrifuged at 2500 X g for 12 min at 4°C. The supernatant was filtered through nylon mesh (pore size, 10μm) and used for biochemical assays [16]
Paraquat Exposure and Concentrations: In a preliminary study, flies were exposed to paraquat at concentrations of 10, 15, 20 and 25 mm for 96h to determine lethality. However, to assess the antioxidant effect of G, only one concentration of Paraquat (15 mM) was employed. For these studies, paraquat exposed flies were provided with G (20 μg/mL) in the diet, the modulatory effect of G was determined on paraquat induced oxidative impairments and locomotors dysfunction.
Typical Exposure Protocol: For mortality studies, a minimum of 50 adult flies per replicate (three replicates) were exposed to G at 20 μg/mL or Paraquat 15 mM or a combination of G+ Paraquat. Lethality of the flies was monitored for 96 h and data was expressed in terms of percent mortality.
Paraquat Resistance Test: Two days after emergence from the pupae, male flies were fed with control food or food containing the compounds for a period of 7 days. Then 50 of the flies fed with the test G, and the control were starved for 6 h to be sure that no food remained in the digestive tract so that none of the compounds would alter the uptake of paraquat. Afterwards, the flies were transferred to vials containing only filter paper soaked with 15 mm paraquat in 5% sucrose solution. This concentration of paraquat was selected because it let us to study the flies for a period of 24h or more after treatment. The survival was determined 24 and 48 h later and the survived flies were used for homogenization for biochemical assays. Each assay was repeated thrice [16].
Assay for Lipid Peroxidation: Lipid peroxidation was measured by employing Thiobarbituric acid (TBA). Briefly, the reaction mixture containing 500 mL fly homogenate, 1.5 mL acetic acid (pH 3.5, 20%), 1.5 mL of TBA (0.8% w/v), 200 mL sodium lauryl sulphate (SDS) (8% w/v). The mixture was heated in a boiling water bath for 45 min and adducts formed were extracted into 3 mL of 1-butanol. The absorbance was measured at 532 nm and quantified as malondialdehyde equivalents using 1,1,3,3- tetra-methoxypropane as the standard [17].
Assay for Hydroperoxide: Hydroperoxide generation was determined according to the standard method, an aliquot of homogenate (100 mg protein) was added to 1 mL of FOX reagent (250 mM Ferrous ammonium sulphate; 100 mM Sorbitol; 25 mM H2SO4; 100 mM Xylenol orange) and incubated for 30 min at room temperature [18]. The absorbance was taken at 560 nm and expressed as nm HP/mg protein.
Estimation of Reduced Glutathione: Reduced glutathione (GSH) content was estimated based on a fluorometric method [19] employing o-phthalaldehyde (OPT). An aliquot of homogenate (stable reduced glutathione with 0.1 M formic acid, 5200 X g for 10 min) was allowed to react with OPT (1 mg/mL in methanol) at room temperature for 30 min and fluorescence measured at excitation of 345 nm and emission at 425 nm.
Activities of Antioxidant Enzymes: Catalase activity was determined according to a reported method. To 1mL reaction mixture containing 8.8mM H2O2 (3%), 0.1mM sodium–phosphate buffer, pH 7.0 the reaction was initiated by adding an aliquot (equivalent to 10 mg protein). The decrease in H2O2 was monitored for 3 min at 240 nm [20]. SOD activity was determined by monitoring the inhibition of quercetin auto oxidation. The total volume of 1 mL reaction mixture containing 3-5 mg protein; 0.016M sodium phosphate buffer, pH 7.8; N,N,N,N Tetramethyl ethylenediamine (TEMED), 8mM and Ethylenediamine tetra acetic acid (EDTA), 0.08mM and reaction was started by adding 0.15% quercetin dissolved in dimethyl formamide. Reaction was monitored for 3 min at 406 nm, expressed as amount of protein required to inhibit 50% of quercetin auto oxidation [21]. Protein estimation was done using flies’ homogenate [22].
Negative Geotaxis Assay: Test flies were anesthetized and placed in a vertical glass column (length, 25 cm; diameter, 1.5 cm) sealed at one end. After a brief recovery period, flies were gently tapped to the bottom of the column. Then, following 1 min, flies that reached to the top of the column and flies that remained at the bottom were counted separately. Data was expressed as percent flies escaped beyond minimum distance of 6 cm in 60s of interval [23,24]. Per replication 20 adults were used for each assay and the assay was repeated three times. The score for each replication was an average of three such trials for each group including control.
Statistical Analysis
Data were expressed as mean SD and all statistical analysis was performed using Prism 3.0 software Comparisons were performed with the One-Way Analysis Of Variance (ANOVA) test. All experiments were performed in triplicates.
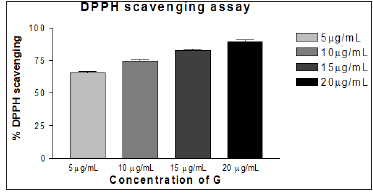
The methanol extract G was characterized physico-chemically and the data on phyto-chemical analysis is illustrated (Tables 1 & 2) respectively. The results reveal that the extract is associated with flavonoids, phenols and other components. The extract G was also screened for their antibacterial activity against Escherichia coli K-12, Staphylococcus aureus, Salmonella typhi, and Bacillus subtilis. It was also screened for their antifungal activity against Aspergillus Niger and Candida albicans. The extract G exhibited significant activity against tested organisms However, based on this promising observation; it is immature to arrive at the conclusion. The zone of inhibition and the Minimum Inhibitory Concentration (MIC) values of the compounds tested against bacteria and fungi are represented (Table 3). The in vitro antioxidant assays like DPPH and TBARS assay were carried out. The extract G exhibited better antioxidant activity in concentration dependent manner and the data is depicted methodically (Figures 2 & 3).
Figure 2: In vitro DPPH scavenging activity of methanol extract of Sida glutinosa (G). Result is expressed as % DPPH• scavenged.
Note: Standard drug used: Bacteria (Ciprofloxacin).
G used : (40 μg in 10mL- based on MIC concentration).
Control : DMSO (dimethyl sulphoxide)
Zone of Inhibition in mm : MIC in μg/mL (data given in the parenthesis is for MIC).
The key objective was to elucidate antioxidant and preliminary neuro-protective efficacy of the G in vivo. The model organism, Drosophila melanogaster was used in this study because it is an excellent model for gerontological research due to its relatively short life span; the adult flies appear to show many of the manifestations of cellular senescence observed in mammals and oxidative stress plays an important role in governing the life span of the fly. The toxicity study was done to determine the lethal concentration of G. Safety evaluation of G revealed that the G was non-toxic at experimental concentration (Table 4).
Different concentrations of paraquat were used to determine concentration and time dependent mortality of flies. The concentration and time dependent mortality response of paraquat on adult male Drosophila melanogaster was given in the (Figure 4). The result also reveals that, there was high mortality (50–60%) among flies during a 7-day exposure period with Par. Whereas, in case of co exposure of G and Par showed significant reduction in mortality rate. The solvent ethanol (100 μl) was kept as solvent control. In this case also mortality rate is insignificant compared to Par treated flies.
Table 4: Lethality Test: Expressed in Percent mortality in Normal flies (Control), Et-OH (Solvent) G at 10,20,40μg/ml concentrations.
It was known that, nigrostriatal dopaminergic neurons are very sensitive to the toxicity of paraquat which can also be toxic to glial cells [25-27]. To determine neuroprotective efficacy of the G geotaxis assay was carried out. Accordingly, the locomotor deficits among flies of various treatment groups employing a similar paradigm. The paraquat treated flies exhibited severe locomotor impairments as evident by the large number of flies staying at the bottom of the glass column, while co-treatment with G significantly improved the performances of flies. With G treatment, a greater number of flies (50-60%) showed negative geotaxis behavior clearly indicating its neuroprotective effect (Figure 5). Incidence of mortality among adult male Drosophila melanogaster exposed to paraquat, is considerably high whereas, there was a decrease incidence of mortality among flies exposed to G + PAR. This results support that G showed protective action (Figure 6). The above results instigated us to determine antioxidant effect of G against paraquat induced oxidative stress.
Figure 4: Concentration and time dependent mortality response among adult male Drosophila melanogaster exposed to PAR in the feed. (n = 50 flies per replicate, three such replication used for assay).
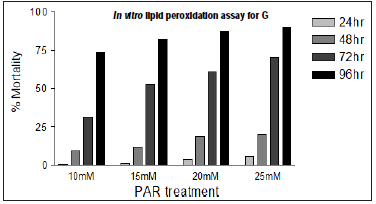
Figure 5: Modulation of paraquat induced locomotor (expressed as percent flies escaped) deficits among adult male Drosophila melanogaster by G (n = 50 flies per replicate, three such replication used for assay. data was analyzed by one-way ANOVA followed by post hoc Tukey test (*p< 0.05).
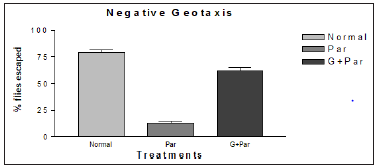
Figure 6: Incidence of mortality among adult male Drosophila melanogaster exposed to Paraquat, co-exposure as G+ PAR and solvent Ethanol (Et-OH); note: decrease incidence of mortality among flies co-exposed with G+ PAR . Values are mean ± SE (in triplicates), data was analyzed by one-way ANOVA followed by post hoc Tukey test (*p < 0.05). Significances were determined by making comparisons between control vs. G, PAR*.
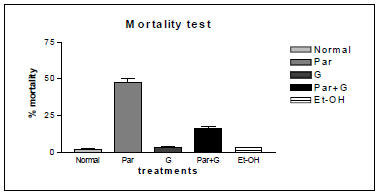
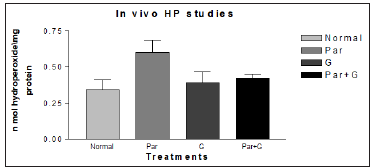
It was also reported that significant induction of oxidative stress among flies exposed to paraquat 15mM evidenced by the marked elevation in MDA and hydroperoxide level coupled with significant change in the activities of antioxidant enzymes such as CAT, SOD which suggested an increased generation of ROS. The ameliorative effects of G on paraquat induced oxidative stress markers in whole body homogenates of adult flies fed with G supplemented diet for 7 days showed significant diminution in MDA and HP levels. The data has been represented analytically (Figures 7 & 8). The reduced GSH is a tripeptide and most abundant soluble antioxidant molecule which plays a crucial role in detoxifying ROS either by reacting directly with radicals nonenzymatically or acting as a substrate in GST catalyzed reactions. Severe depletion in cellular GSH levels upon paraquat exposure in Drosophila adds further evidence that a state of oxidative stress exists in vivo which may lead to mitochondrial damage, increase in free radical generation and peroxidation of membrane lipids. Although paraquat exposure for 7 days caused significant decrease in GSH level, on co exposure with G, flies were able to restore the depleted GSH levels significantly (Figure 9) clearly suggesting the ability of G to up regulate levels of GSH.
Figure 7: Ameliorative effect of G treatment on paraquat (PAR) induced oxidative stress measured as malondialdehyde levels in whole body homogenates of adult Drosophila melanogaster. Where (p* < 0.05,) for the group treated with Par* compared with normal. Par+G* compared with Par.
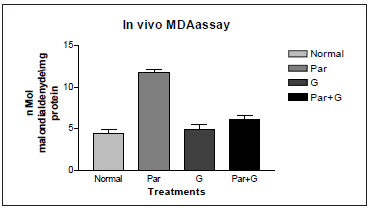
Figure 8: Ameliorative effect of G treatment on Paraquat (PAR) induced oxidative stress measured as hydroperoxide levels in whole body homogenates of adult Drosophila melanogaster. Where (p* < 0.05,) for the groups treated with Par+ G* compared with Par.
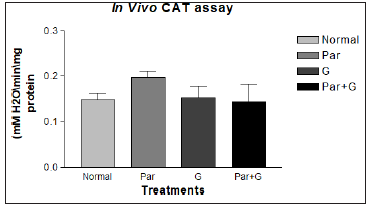
Paraquat (15 mM) exposure induced a significant elevation in the activity of all antioxidant enzymes measured. However, among G + paraquat exposed flies, the activity levels of SOD and CAT, were restored to near basal levels, (Figures 10 & 11). Based on biochemical evidences, it is proposed that dietary feeding of leaf extract of Sida glutinosa (G) to Drosophila for a short duration has the propensity to attenuate paraquat induced oxidative stress owing to its antioxidative nature and its ability to modulate the activities of antioxidant defenses such as reduced GSH and antioxidant defenses. The additional evidences viz., lower incidence of paraquat induced mortality and higher resistance to paraquat among flies pretreated with G. Further, its antioxidant property was clear by its ability to significantly abrogate paraquat induced oxidative stress, by depleting the lipid peroxidation product malondialdehyde.
Figure 9: Ameliorative effect of G treatment on Paraquat (PAR) induced oxidative stress measured as reduced glutathione levels in whole body homogenates of adult Drosophila melanogaster. Where, (p* < 0.05,) for the groups treated with G* and Par+ G* in comparison with normal (control) and Par.
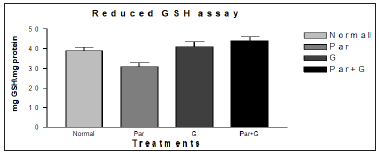
Figure 10:Modulatory effect of G on the endogenous markers of oxidative stress SOD in adult Drosophila melanogaster. No significant change in the level of SOD found the groups treated with G but restored to near basal level compared to Normal (control) and Par+ G* with Par.
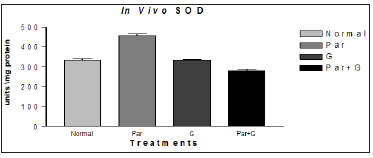
Figure 11: Modulatory effect G on the endogenous markers of oxidative stress HP in adult Drosophila melanogaster. Significances were determined by making comparisons between control vs treated group. Where (*p < 0.05) for the groups treated for G* with the Normal (control) group and Par+ G* with Par.
The results obtained in ‘Paraquat Resistance Assay’ suggest that G prophylaxis has the propensity to protect against neurotoxicant exposure largely due to its antioxidative potential. In this model, the protective activity of G was highly comparable to paraquat alone treated. More importantly this data further confirms the utility value of Drosophila system as a primary model to rapidly screen potential compounds for their antioxidant properties prior to their testing in mammalian models and final therapeutic use in humans.
It is evident from the experimental results that, the leaf extract of Sida glutinosa G is showing good antioxidant, antimicrobial against tested organisms and neuroprotective agent in Drosophila model system. This work opens an avenue for further investigation towards the isolation characterization of active principle and evaluation of biological properties in higher model system. In addition, the biological activity of this ethno-medicinal plant justifies its practice by tribal community at B.R. Hills. Further, the active principle needs to be characterized and the lead molecule must be confirmed using specific biophysical techniques.


