Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jui-Chih Chang*1, Yi-Chun Chao1, Huei-Shin Chang1, Hui-Ju Chang1, Wen-Ling Cheng1, Ta-Tsung Lin1 and Chin-San Liu*1,2,3
Received: November 30, 2018; Published: December 06, 2018
*Corresponding author: Jui-Chih Chang, Department of Vascular and Genomic Center, Changhua Christian Hospital, 135 Nanhsiao Street, Changhua 50094, Taiwan
Chin-san Liu, Department of Neurology, Changhua Christian Hospital, 135 Nanhsiao Street, Changhua 50094, Taiwan
DOI: 10.26717/BJSTR.2018.11.002158
Abbrevations: PD: Parkinson’s Disease; OB: Olfactory Bulb; SN: Substantia Nigra; BBB: Blood–Brain Barrier; CNS: Central Nervous System; CSF: Cerebrospinal Fluid; BCSFB: Blood-CSF Barrier; DA: Dopamine; D0PAC: Di Hydroxy Phenyl Acetic Acid; CPP: Cell Penetrating Peptide
Olfactory dysfunction has been recently identified as one of the earliest non-motor symptoms of Parkinson’s disease (PD); it occurs at an early stage in approximately 90% of patients with PD and can be observed several years before the onset of limb disorders. Although the mechanism of olfactory dysfunction and its association with PD remain unclear, same symptoms have been observed in patients with Alzheimer disease and Down syndrome. Therefore, it is confirmed that the close association between the olfactory bulb (OB) and cranial nerves plays an important role in neurodegeneration [1]. The OB affects the function of dopaminergic neurons. Recent studies have demonstrated that the repeat unilateral intra-nasal administration (i.n.) of the mitochondrial inhibitor rotenone for 3-7 days can damage dopaminergic neurons in both sides of the OB, thereby resulting in olfactory dysfunction; the inhibitory effect is more pronounced in the i.n. side. Therefore, the result suggests that rotenone affects neuronal function via olfactory transport [2].
Meanwhile, unilateral i.n. induces dopaminergic neuron damage in both sides of the OB, indicating that neurotoxins spread to the brain via olfactory transport [2]. Therefore, i.n. may regulate brain cell functions, such as the mitochondrial functions of dopaminergic neurons via olfactory transport. Further studies confirming whether the i.n. of neurotoxins can chronically induce dopaminergic neuron death in the substantia nigra (SN) are warranted [3]; however, most studies have suggested that the delivery of inhaled environmental toxins via olfactory transport is one of the main inducers of spontaneous neurodegenerative diseases such as PD [4].
The development of effective genetic or drug therapy for PD has always been a hotspot in research. However, the efficacy of treatments is limited mainly because the delivery of therapeutic substances is hampered due to the inability of large biomolecules to cross the blood–brain barrier (BBB). One solution is to perform intra-cerebral infusion directly at the location of the lesion. In recent years, studies have confirmed the efficacy of the i.n. of nanomedicines in the treatment of PD [5]. A study found that i.n. is an effective route, which not only reduces brain tissue damage as a non-surgical method but also provides a new way to bypass the BBB via the olfactory and trigeminal neural pathways, which connect the nasal mucosa to the perivascular pathways in the brain and central nervous system (CNS) [6]. Simultaneously, the i.n. route bypasses the cardiovascular system, reduces the metabolism of drugs in the liver and increases drug utilisation, thereby providing an effective method for non-invasive drug delivery to the CNS [7]. In addition to the olfactory and trigeminal neural pathways, the study have also found that the i.n. of drugs or neurotransmitters allows these drugs to cross the nasal cavity, enter the cerebrospinal fluid (CSF) and be directly transported to the brain via the blood–CSF barrier (BCSFB) [8]. Unlike the BBB, which is formed by endothelial cells, BCSFB is located at the cerebral choroid plexus and comprises epithelial cells. Its functions are similar to that of the BBB, but with lower selectivity and higher permeability than the BBB to maintain CSF production by eliminating waste, maintaining neurotransmitter homeostasis and accelerating intra-cerebral substance transfer [8].
The nose-to-brain pathway enables the rapid delivery of therapeutic agents to the CNS in minutes. The rapid delivery of small and highly lipophilic molecules, whether genetic or proteinbased, into the CNS was found to have the higher efficacy via i.n. [9]. Mattern, C. et al. encapsulated the neurotransmitter dopamine (DA) in a highly lipophilic gel and used it in the treatment of PD in rats via i.n., where the encapsulation enables a regulated release of DA [9]. Their results showed that DA concentrations in the nucleus accumbens and new striatum of the rat brain increased very rapidly, thereby reaching a level that was 2-fold higher than that in the rats treated with DA aqueous solution. Moreover, DA presence was detected in the OB, peaking at 4 hours after its administration; however, the DA presence in the treatment of non-encapsulated DA was much lower than that in treatment of lipid-encapsulated DA. In addition, DA in the CSF had not been metabolised into 3,4-dihydroxyphenylacetic acid (D0PAC) or high vanillic acid as observed normally, indicating that the use of encapsulated DA improves the efficacy of nasal DA administration in the treatment of diseases associated with DA deficiency in the brain, such as PD, attention deficit hyperactivity disorder or drug and/or alcohol addiction [9,10].
The encapsulation of drugs with colloids or nanoparticles can:
a) Protect drugs from degradation during their delivery;
b) Increase drug absorption via the extracellular transfer protein P-glycoprotein and
c) Help the drug cross the olfactory epithelium. Whether the drug enters the brain via the BCSFB or trigeminal neural pathways remains unclear
d) Taken together, the successful drug administration via the nose-to-brain pathway depends on the chemical and physical properties of drugs, such as size, adhesion, stability, hydrophilicity and penetrating power. Compared with nasal inhalation, which involves the lung track, the use of nasal drops or ointments is simpler in terms of the i.n. of drugs that directly regulate brain cell function.
Mitochondria are nano-sized cell organelles; the mitochondrion of a eukaryotic cell is approximately 200-1000 nm in size. Recent animal studies have found that the in situ or intravenous injection of mitochondria isolated from healthy cells can be used in the treatment of diseases caused by mitochondrial damage. Although in vivo unmodified mitochondria may enter cells via interstitial pressure, osmosis or endocytosis [11, 12], the in vitro experiments performed by our research team have demonstrated that cells with mitochondrial damage, such as myoclonus epilepsy with ragged-red fibres (MERRF) syndrome, have disrupted cytoskeleton functions, thereby resulting in a decreased probability of the mitochondria entering cells via actin-dependent endocytosis [12]. To improve the efficacy of mitochondria entering the damaged cells, our team has previously studied the modification of isolated mitochondria with Pep-1, one of cell penetrating peptide (CPP) family.
Experiments on cells with mitochondrial diseases [MERRF and mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke (MELAS)] [13-15] as well as animal tests in rats with PD [16] confirmed that the Pep-1-labelled mitochondria can move to the locations of damaged cell mitochondria and increase the import of mitochondria by the damaged cells to restore mitochondrial function and reduce oxidative stress. The related mechanism is associated with the inhibition of ERK phosphorylation, decrease of matrix metalloproteinases (MMP1) activation and restoration of actin cytoskeleton upstream regulatory protein cdc42 GTPase [17]. Therefore, in our recent studies we used rats with 6-OHDA drug-induced PD as models to investigate and establish a noninvasive mitochondrial transplantation system for the treatment of PD via the nose-to-brain pathway. We also explored whether Pep-1-labelling accelerates the speed of mitochondria that enter the olfactory (olfactory transport) or dopaminergic nerves through the CSF via the BCSFB and are transported to the dopaminergic neuron bodies to repair the mitochondrial function. In our previous experiments, BrdU-labelled homologous hepatic mitochondria solutions, with or without Pep-1 modification, were administrated via unilateral nasal infusion at the side of cerebral injury once a week for 3 months.
Figure 1:Expression of foreign mitochondria labelled with BrdU-labelled was observed in OB tissue (as the brown color shown) after three month grafts in PD rats. Scale bar 100 μm. Abbr. Mito, mitochondrial alone; P-Mito, Pep-1- labelled mitochondria.
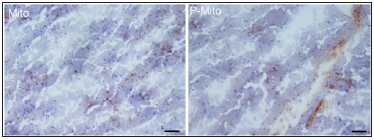
The results demonstrated that BrdU-labelled foreign mitochondria were observed both in the OB tissue and striatum (Figure 1). Mitochondria transplantation, with or without Pep- 1 modification, reduced the amphetamine-induced rotational behaviours in rats with PD. The locomotive activity of the rats, including the moving distance and rate, was also improved. The Pep-1-labelled mitochondria group (P-Mito) showed more significant improvement in locomotive activity than the Mito group [18]. Currently, the relevant pathological tissue section analysis is still in progress, including SN neuronal activity, mitochondrial function assessment, oxidative stress and the staining analysis of tyrosine hydroxylase, a marker for dopaminergic neurons. Recent studies have in fact demonstrated that stem cells may enter the CNS after i.n. [19] and can be used in the treatment of PD [20] and brain malignant tumours [21]; however, whether this approach is applicable to mitochondria transplantation remains unclear. Our preliminary study demonstrates the potential of nasal mitochondria delivery in treating PD as it can avoid the risk of surgery and side effects caused by invasive non-pharmacological transplantation.


